CHROMITOPE SODIUM
-
sodium chromate cr-51 injection, solution
BRACCO DIAGNOSTICS INC.
----------
DESCRIPTION
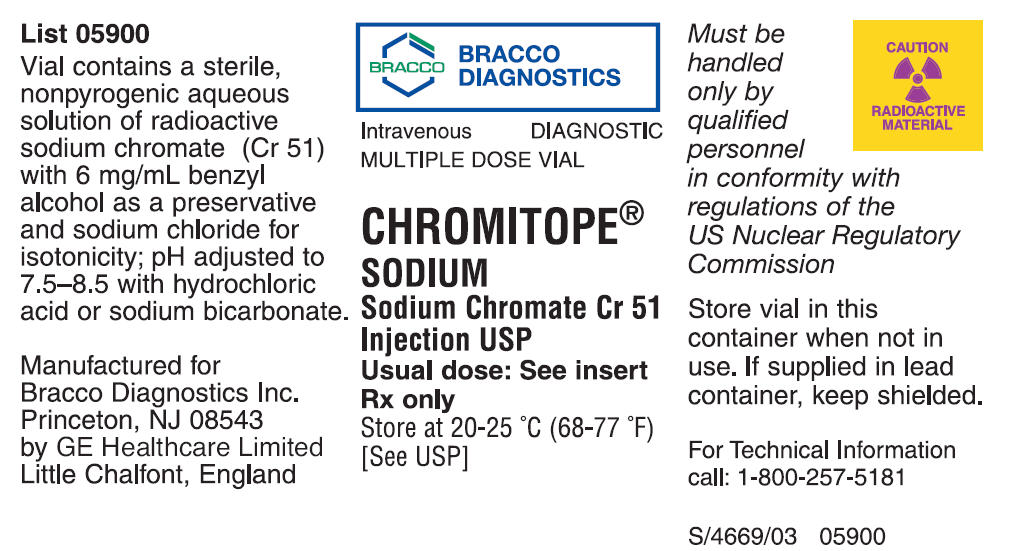
Chromitope Sodium (Sodium Chromate Cr 51 Injection USP) is a diagnostic radiopharmaceutical for intravenous administration. This agent provides radioactive chromium 51 in the form of sterile, nonpyrogenic aqueous solution of sodium chromate (Na2 51CrO4). The solution also contains 6 mg/mL benzyl alcohol as a preservative and sodium chloride for isotonicity; the pH has been adjusted to 7.5–8.5 with hydrochloric acid or sodium bicarbonate.
PHYSICAL CHARACTERISTICS
Chromium 51 decays by electron capture and gamma emission with a physical half-life of 27.7 days.1 The principal photon that is useful for detection and imaging studies is listed in Table 1.
| Principal Radiation Emission Data | ||
|---|---|---|
| Radiation | Mean % per Disintegration | Mean Energy (keV) |
| Gamma-1 | 9.83 | 320.1 |
External Radiation
The specific gamma ray constant for Cr 51 is 0.19 R/hour-millicurie at 1 cm. The first half-value layer is 0.20 cm lead (Pb). Values for the relative attenuation of the radiation emitted by the radionuclide that result from interposition of two thicknesses of Pb are shown in Table 2. To facilitate control of the radiation exposure from millicurie amounts of this radionuclide, the use of a 0.60 cm thickness of lead will attenuate the radiation emitted by a factor of about 10.
| Radiation Attenuation by Lead Shielding | |
|---|---|
| Shield Thickness (Pb) cm | Attenuation Factor |
| 0.20 | 0.5 |
| 0.60 | 10-1 |
To correct for physical decay of CR 51, the fractions that remain at selected intervals before and after the time of calibration are shown in Table 3.
| Physical Decay Chart: Cr 51 half-life 27.7 days | |||||
|---|---|---|---|---|---|
| *Calibration time | |||||
| Days | Fraction Remaining | Days | Fraction Remaining | Days | Fraction Remaining |
| 0* | 1.000 | 25 | 0.535 | 60 | 0.223 |
| 1 | 0.975 | 30 | 0.472 | 65 | 0.197 |
| 2 | 0.975 | 35 | 0.417 | 70 | 0.173 |
| 4 | 0.905 | 40 | 0.368 | 75 | 0.153 |
| 8 | 0.819 | 45 | 0.324 | 80 | 0.135 |
| 15 | 0.687 | 50 | 0.286 | 85 | 0.119 |
| 20 | 0.606 | 55 | 0.253 | ||
CLINICAL PHARMACOLOGY
The chromium in this agent is present as the dianionic chromate ion in which form it appears to bind to the red blood cell in two steps, initially by a rapid but reversible attachment to the cell membrane followed by a slower nearly irreversible binding to intracellular hemoglobin and reduction to the anionic state. It has been suggested that the slow rate of uptake is dependent on the rate at which chromate can penetrate the cell membrane. Binding is maintained until the red blood cells are sequestered by the spleen or until elution of the chromium occurs into the plasma. The chromium is then readily excreted mainly in the urine. Once liberated by elution or erythrocyte senescence, chromium 51 is not available for relabeling of red cells.
In normal individuals the erythrocyte survival half-time (T ½) as measured by the chromium 51 “random labeling” technique, generally ranges between 25 and 35 days. This apparent short survival time, when compared to the 120 day life span of the red blood cells, is due to the elution of chromium from the cells and to cell damage that probably occurs during the process of withdrawing them from the body and labeling. Subnormal T 1/2 may be indicative of blood loss, sequestration of red blood cells by the spleen, or shortened cell viability, as occurs in hemolytic anemia.
INDICATIONS AND USAGE
Chromitope Sodium (Sodium Chromate Cr 51 Injection USP) is indicated for use in determining red blood cell volume or mass, studying red blood cell survival time (in conditions such as hemolytic anemia), and evaluating blood loss.
PRECAUTIONS
General
In the use of any radioactive material, care should be taken to insure minimum radiation exposure to the patient and occupational workers consistent with proper patient management.
Nuclear medicine procedures involving withdrawal and reinjection of blood have the potential for transmission of blood borne pathogens. Procedures should be implemented to avoid administration errors and viral contamination of personnel during blood product labeling. A system of checks similar to the ones used for administering blood transfusions should be routine.
In order to obviate or minimize the possibility of contamination and of increased fragility of the labeled red blood cells, sterile techniques should be employed throughout the collection, labeling, rinsing, suspending, and injection of red blood cells. In addition, the number of washes and transfers should be kept to a minimum and only sterile, non-pyrogenic isotonic diluent should be employed throughout the labeling procedure.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic or mutagenic potential, or whether this agent may impair fertility in males or females.
Pregnancy: Teratogenic Effects
Category C
Animal reproduction studies have not been conducted with Sodium Chromate Cr 51 Injection. It is also not known whether this agent can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. A suspension of chromium 51-labeled red blood cells containing Sodium Chromate Cr 51 Injection and Bracco A-C-D Solution Modified (Anticoagulant Citrate Dextrose Solution Modified) should be administered to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, of a woman of childbearing capability should be performed during the first few (approximately 10) days following the onset of menses.
ADVERSE REACTIONS
No adverse reactions specifically attributable to Sodium Chromate Cr 51 Injection have been reported.
DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The usual doses to the average patient (70 kg) are as follows: Determination of red blood cell volume or mass–0.37 to 1.11 megabecquerels (10 to 30 microcuries).
Study of red blood cell survival time–5.55 megabecquerels (150 microcuries).
Evaluation of blood loss–7.40 megabecquerels (200 microcuries).
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
The specific activity should be not less than 370 megabecquerels (10 millicuries) per mg [0.37 megabequerels (10 microcuries) per µg] of Sodium Chromate at the time of use.
It is essential that the user adhere to strict aseptic procedures during the preparation, withdrawal and administration of the labeled red blood cells. Waterproof gloves are to be worn during the labeling procedure to prevent the possibility of radioactive contamination of the hands. Shielded syringes should be used when adding the Chromitope Sodium to the reaction vial and for the withdrawal and administration of the labeled red blood cells. To maintain adequate shielding during the life of the labeled preparation, a lead vial shield and lead cover must remain in place on the reaction vial.
Red Blood Cell Labeling Procedure
Labeling may be performed without washing or centrifugation steps directly in the silicone-coated reaction vial of Bracco A-C-D Solution Modified.*
*Anticoagulant Acid Citrate Dextrose Solution Modified containing the same ingredients as Anticoagulant Citrate Dextrose Solution USP but in different ratio (see package insert for complete details). Bracco A-C-D Solution Modified is supplied as 10 mL of sterile, nonpyrogenic solution in convenient silicone-coated 75 mL capacity reaction vials.
A 30 to 50 mL sample of whole blood is withdrawn from the patient and added aseptically to a vial of Bracco A-C-D Solution Modified. 1.85 to 5.55 megabecquerels (50 to 150 microcuries) of Chromitope Sodium (Sodium Chromate Cr 51 Injection USP) is then injected into the reaction vial using a shielded syringe. The amount of radioactivity added to the vial will depend on the intended use of the labeled red blood cells. The suspension is incubated for 30 to 60 minutes at room temperature with frequent gentle agitation. After incubation, 100 mg Ascorbic Acid Injection USP is injected into the vial. The ascorbic acid reduces any remaining unbound dianionic chromium 51 to the anionic state which does not penetrate red blood cells; thus in vivo labeling of red blood cells is prevented.
The extent of chromium 51 labeling is influenced by hematocrit values; hematocrits below 35 percent will result in a higher degree of labeling, whereas hematocrits exceeding 45 percent will produce the opposite result. Although the rate of labeling is initially more rapid at 37° C, samples incubated at 37° C and room temperature will show the same degree of chromium 51 labeling after a 30-minute period.
Labeling may also be accomplished by centrifugation and washing techniques which are described in the literature.
The admixture of Sodium Chromate Cr 51 Injection with Bracco A-C-D Solution Modified (Anticoagulant Citrate Dextrose Solution Modified) for use as a stock solution is not recommended. For best results, the whole blood sample should be combined with Bracco A-C-D Solution Modified prior to labeling.
Radiation Dosimetry
The estimated absorbed radiation doses to an average patient (70 kg) following an intravenous injection of 7.40 megabecquerels (200 microcuries) of Cr 51 are shown in Table 4.
| Estimated Absorbed Radiation Doses | ||
|---|---|---|
| Method of Calculation: A Schema for Absorbed-Dose Calculations for Biologically Distributed Radionuclides, MIRD Pamphlet No. 1. J Nucl Med Suppl 1:7, 1968 | ||
| Tissue | mGy/ 7.40 MBq | rads/ 200 µCi |
| Blood | 2.0 | 0.20 |
| Spleen | 26.4 | 2.64 |
| Testes | 0.66 | 0.066 |
| Ovaries | 0.66 | 0.066 |
| Whole body | 0.55 | 0.055 |
HOW SUPPLIED
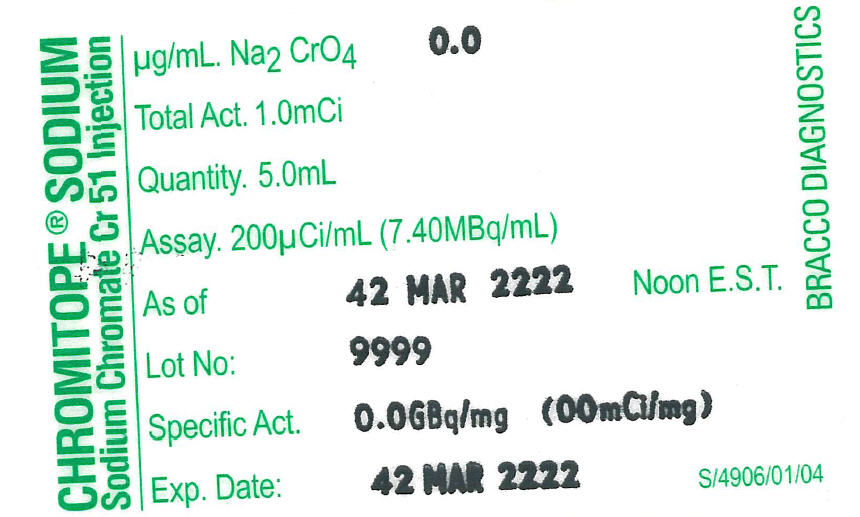
Chromitope Sodium (Sodium Chromate Cr 51 Injection USP) is available in multiple dose vials containing 9.25 megabecquerels (250 microcuries) (1.25 mL) and 37 megabecquerels (1.0 millicurie) (5.0 mL) at the time of calibration. Complete assay data for each vial are provided on the container.
Disposal
Any unused portion of the labeled preparation must be stored and disposed of in accordance with the conditions of NRC radioactive material license pursuant to Agreement State Regulation.
The U.S. Nuclear Regulatory Commission has approved this prepared radiopharmaceutical for distribution to persons licensed to use byproduct material identified in §35.100 of 10 CFR Part 35, to persons who hold an equivalent license issued by an Agreement State, and, outside the United States, to persons authorized by the appropriate authority.
Manufactured for
Bracco Diagnostics Inc.
Princeton, NJ 08543
by GE Healthcare Limited
Little Chalfont, England
Revised August 2005
S/4669/03
| CHROMITOPE SODIUM
sodium chromate, cr 51 injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA013993 | 12/27/1971 | 09/01/2012 |
| Labeler - BRACCO DIAGNOSTICS INC. (849234661) |
| Registrant - BRACCO DIAGNOSTICS INC. (849234661) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GE HEALTHCARE LTD | 217152818 | MANUFACTURE | |
Revised: 05/2012 BRACCO DIAGNOSTICS INC.