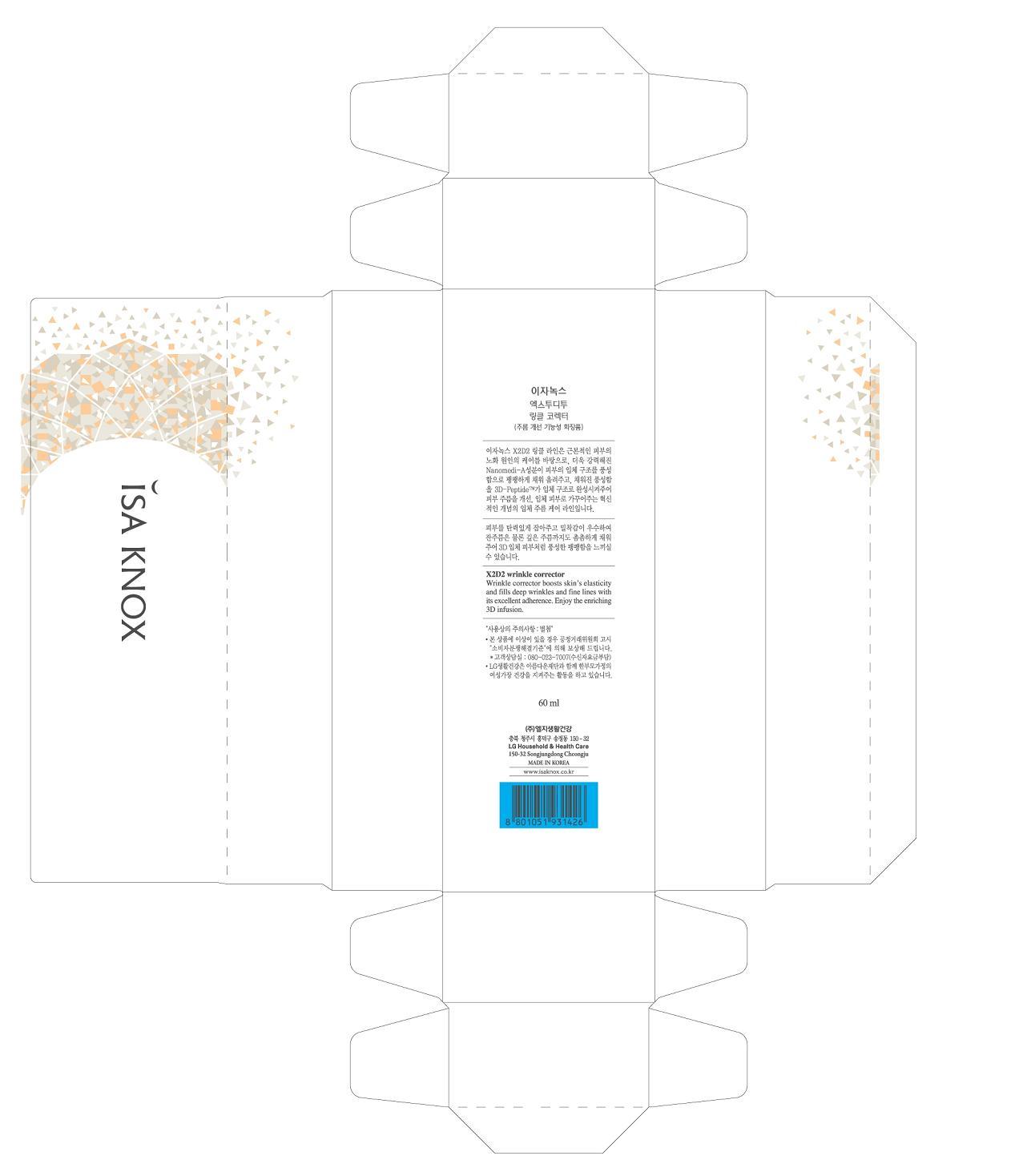
ISA KNOX X2D2 WRINKLE CORRECTOR
-
methoxy peg-12 retinamide cream
LG Household and Healthcare, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
METHOXY PEG-12 RETINAMIDE 0.1125%
Keep out of reach of children. Is swallowed, get medical help or contact a Poison Control Center right away.
Wrinkle functional cosmetic.
Keep out of eyes. Rinse with water to remove.
After applying emulsion, apply a pea-sized amount over the area of concern. For topical treatment around the eyes, take a pea-sized amount and lightly tap on for better absorption.
WATER, SHEA BUTTER, BUTYLENE GLYCOL, GLYCERIN, DIPROPYLENE GLYCOL, GLYCERYL STEARATE, PANTHENOL, FRUIT, DIMETHICONE, ALANYL-GLUTAMINE, SILICON, POLYOXYL 40 STEARATE, PHENOXYETHANOL, POTASSIUM HYDROXIDE, PROPYLPARABEN, ETHYLPARABEN, ALPHA-TOCOPHEROL, EDETATE TRISODIUM.
ISA KNOX X2D2 WRINKLE CORRECTOR
ISA KNOX X2D2 WRINKLE CORRECTOR
methoxy peg-12 retinamide cream |
|
|
|
|
|
|
|
|
|
|
Revised: 05/2012 LG Household and Healthcare, Inc.
