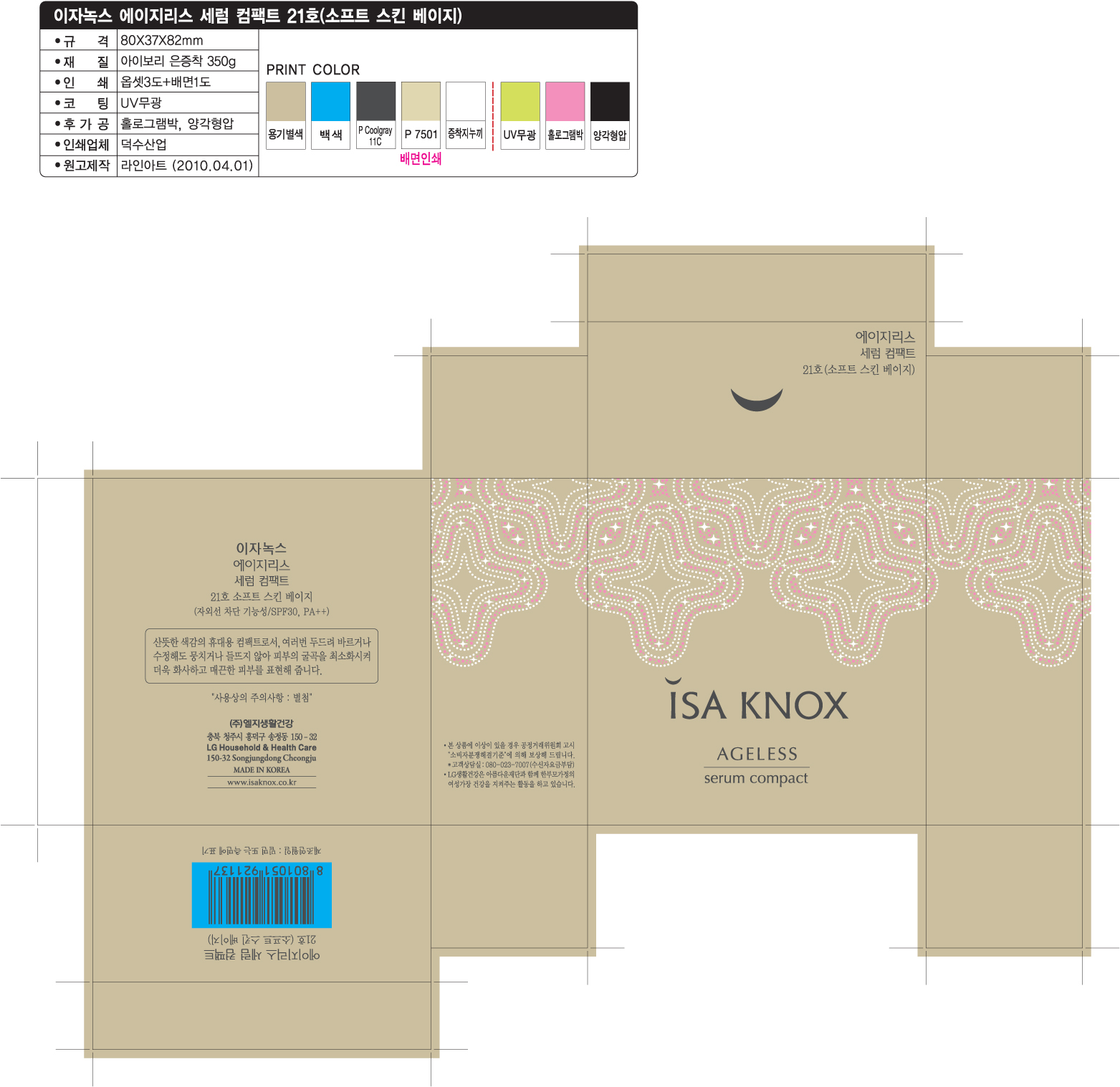
ISAKNOX AGELESS SERUM COMPACT 21
-
titanium dioxide and
octinoxate powder
LG Household and Healthcare, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
TITANIUM DIOXIDE 4.9g/100g
OCTINOXATE 2g/100g
Warning and Precautions
For external use only.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. Is swallowed, get medical help or contact a Poison Control Center right away.
WHEN USING
Keep out of eyes. Rinse with water to remove.
Isaknox
Ageless
Serum Compact 21
ISAKNOX AGELESS SERUM COMPACT 21
titanium dioxide, octinoxate powder |
|
|
|
|
|
|
|
|
|
|
Revised: 05/2012 LG Household and Healthcare, Inc.
