|
|
|||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
Asacol is a delayed-release aminosalicylate indicated for:
- the treatment of mildly to moderately active ulcerative colitis.
- the maintenance of remission of ulcerative colitis.
2. DOSAGE AND ADMINISTRATION
2.1. For the treatment of mildly to moderately active ulcerative colitis:
The recommended dose in adults is 2.4 g/day or 4.8 g/day (two or four 400mg tablets three times a day). Treatment duration in controlled clinical trials was 6 weeks.
2.2 For the maintenance of remission of ulcerative colitis:
The recommended dosage in adults is 1.6 grams daily, in divided doses. Treatment duration in the prospective, well-controlled trial was 6 months.
3. DOSAGE FORMS AND STRENGTHS
Asacol tablets are available as red-brown, capsule-shaped tablets containing 400 mg mesalamine and imprinted “Asacol NE” in black.
4. CONTRAINDICATIONS
Asacol tablets are contraindicated in patients with hypersensitivity to salicylates or to any of the components of the Asacol tablet.
5. WARNINGS AND PRECAUTIONS
5.1 Renal
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis, and, rarely, renal failure has been reported in patients taking Asacol tablets as well as other compounds which contain or are converted to mesalamine. In animal studies (rats, dogs), the kidney is the principal target organ for toxicity. At doses of approximately 750 mg/kg to 1000 mg/kg [15 to 20 times the administered recommended human dose (based on a 50 kg person) on a mg/kg basis and 3 to 4 times on a mg/m2 basis], mesalamine causes renal papillary necrosis. Therefore, caution should be exercised when using Asacol (or other compounds which contain or are converted to mesalamine or its metabolites) in patients with known renal dysfunction or history of renal disease. It is recommended that all patients have an evaluation of renal function prior to initiation of Asacol tablets and periodically while on Asacol therapy.
5.2 General
Exacerbation of the symptoms of colitis has been reported in 3% of Asacol-treated patients in controlled clinical trials. This acute reaction, characterized by cramping, abdominal pain, bloody diarrhea, and occasionally by fever, headache, malaise, pruritus, rash, and conjunctivitis, has been reported after the initiation of Asacol tablets as well as other mesalamine products. Symptoms usually abate when Asacol tablets are discontinued.
Some patients who have experienced a hypersensitivity reaction to sulfasalazine may have a similar reaction to Asacol tablets or to other compounds which contain or are converted to mesalamine.
Patients with pyloric stenosis may have prolonged gastric retention of Asacol tablets which could delay release of mesalamine in the colon.
6. ADVERSE REACTIONS
Renal impairment, including renal failure (rare) has been reported. (See Warnings and Precautions ( 5.1))
Acute exacerbation of ulcerative colitis can occur. (See Warnings and Precautions ( 5.2))
6.1 Adverse Events from Clinical Trials
Asacol tablets have been evaluated in over 4000 inflammatory bowel disease patients (most patients with ulcerative colitis) in controlled and open-label studies. Adverse events seen in clinical trials with Asacol tablets have generally been mild and reversible. Adverse events presented in the following sections may occur regardless of length of therapy and similar events have been reported in short- and long-term studies and in the post-marketing setting.
In two short-term (6 weeks) placebo-controlled clinical studies involving 242 patients, 155 of whom were randomized to Asacol tablets, five (3.2%) of the Asacol patients discontinued Asacol therapy because of adverse events as compared to two (2.3%) of the placebo patients. Adverse reactions leading to withdrawal from Asacol tablets included (each in one patient): diarrhea and colitis flare; dizziness, nausea, joint pain, and headache; rash, lethargy and constipation; dry mouth, malaise, lower back discomfort, mild disorientation, mild indigestion and cramping; headache, nausea, aching, vomiting, muscle cramps, a stuffy head, plugged ears, and fever.
Adverse events occurring in 3% or greater of Asacol-treated patients in the two short-term, double-blind, placebo-controlled trials mentioned above are listed in Table 1 below. Overall, the incidence of adverse events seen with Asacol tablets was similar to placebo.
|
N = number of patients within specified treatment. |
|||
|
n = number of patients in category and treatment group. |
|||
|
% =percentage of patients in category and treatment group: (n/N)*100. |
|||
| 2.4 g/day | 4.8 g/day | ||
| Placebo | (400 mg tablet) | (400 mg tablet) | |
| (N=87) | (N=53) | (N=38) | |
| COSTART | n (%) | n (%) | n (%) |
| HEADACHE | 31 (36%) | 23 (43%) | 6 (16%) |
| ERUCTATION | 13 (15%) | 14 (26%) | 2 (5%) |
| ABDOMINAL PAIN | 12 (14%) | 11 (21%) | 3 (8%) |
| CONSTIPATION | 1 (1%) | 6 (11%) | 0 (0%) |
| NAUSEA | 13 (15%) | 6 (11%) | 6 (16%) |
| PHARYNGITIS | 8 (9%) | 6 (11%) | 3 (8%) |
| DIZZINESS | 7 (8%) | 5 (9%) | 2 (5%) |
| RHINITIS | 4 (5%) | 4 (8%) | 3 (8%) |
| BACK PAIN | 4 (5%) | 3 (6%) | 0 (0%) |
| RASH | 3 (3%) | 3 (6%) | 3 (8%) |
| ARTHRALGIA | 3 (3%) | 2 (4%) | 2 (5%) |
| CHILLS | 2 (2%) | 2 (4%) | 1 (3%) |
| DYSPEPSIA | 1 (1%) | 2 (4%) | 1 (3%) |
| FLU SYNDROME | 2 (2%) | 2 (4%) | 1 (3%) |
| PAIN | 7 (8%) | 2 (4%) | 9 (24%) |
| CHEST PAIN | 2 (2%) | 2 (4%) | 1 (3%) |
| SWEATING | 1 (1%) | 2 (4%) | 0 (0%) |
| ULCERATIVE COLITIS | 0 (0%) | 1 (2%) | 2 (5%) |
| VOMITING | 2 (2%) | 1 (2%) | 2 (5%) |
| ACNE | 1 (1%) | 0 (0%) | 2 (5%) |
| HEMATURIA | 0 (0%) | 0 (0%) | 2 (5%) |
| TREMOR | 0 (0%) | 0 (0%) | 2 (5%) |
Two six-week, randomized, double-blind, active-controlled studies (ASCEND I and II), evaluated Asacol 2.4 g/day (400mg tablet) and 4.8 g/day (800mg tablet) in 687 patients with mildly to moderately active ulcerative colitis. The most commonly reported adverse events were headache and GI symptoms, including abdominal pain, diarrhea, nausea, vomiting, flatulence, and dyspepsia. In these studies, therapy with 4.8 g/day had a safety profile that was comparable to Asacol 2.4 g/day and was not associated with an increased incidence of adverse events.
In a 6-month placebo-controlled maintenance trial involving 264 patients, 177 of whom were randomized to Asacol tablets, six (3.4%) of the Asacol patients discontinued Asacol therapy because of adverse events, as compared to four (4.6%) of the placebo patients. Adverse reactions leading to withdrawal from Asacol tablets included (each in one patient): anxiety; headache; pruritus; decreased libido; rheumatoid arthritis; and stomatitis and asthenia.
Adverse events occurring in 3% or greater of Asacol-treated patients in the 6-month double-blind, placebo-controlled maintenance study mentioned above are listed in Table 2 below. Overall, the incidence of adverse events seen with Asacol tablets was similar to that seen with placebo.
|
N = number of patients within specified treatment. |
||
|
n = number of patients in category and treatment group. |
||
|
% =percentage of patients in category and treatment group: (n/N)*100. |
||
| 1.6 g/day | ||
| Placebo | (400 mg tablet) | |
| (N=87) | (N=87) | |
| COSTART | n (%) | n (%) |
| RHINITIS | 31 (36%) | 35 (40%) |
| PAIN | 10 (11%) | 20 (23%) |
| PHARYNGITIS | 13 (15%) | 18 (21%) |
| ASTHENIA | 14 (16%) | 17 (20%) |
| NAUSEA | 13 (15%) | 15 (17%) |
| FEVER | 11 (13%) | 12 (14%) |
| ULCERATIVE COLITIS | 7 (8%) | 9 (10%) |
| GASTROINTESTINAL HEMORRHAGE | 7 (8%) | 9 (10%) |
| ABNORMAL STOOLS | 7 (8%) | 9 (10%) |
| INFECTION | 3 (3%) | 8 (9%) |
| DIZZINESS | 6 (7%) | 7 (8%) |
| MYALGIA | 4 (5%) | 7 (8%) |
| CHEST PAIN | 5 (6%) | 7 (8%) |
| RECTAL DISORDER | 2 (2%) | 6 (7%) |
| SINUSITIS | 5 (6%) | 6 (7%) |
| TENESMUS | 4 (5%) | 6 (7%) |
| NERVOUSNESS | 2 (2%) | 5 (6%) |
| DYSMENORRHEA | 2 (2%) | 4 (5%) |
| GASTROENTERITIS | 1 (1%) | 4 (5%) |
| HYPERTONIA | 3 (3%) | 4 (5%) |
| MALAISE | 3 (3%) | 4 (5%) |
| HEMATURIA | 1 (1%) | 3 (3%) |
| JOINT DISORDER | 0 (0%) | 3 (3%) |
| LUNG DISORDER | 0 (0%) | 3 (3%) |
| URINARY FREQUENCY | 0 (0%) | 3 (3%) |
| ABNORMAL VISION | 0 (0%) | 3 (3%) |
In 3342 patients in uncontrolled clinical studies, the following adverse events occurred at a frequency of 5% or greater and appeared to increase in frequency with increasing dose: asthenia, fever, flu syndrome, pain, abdominal pain, back pain, flatulence, gastrointestinal bleeding, arthralgia, and rhinitis.
6.2 Adverse Events from Other Sources
In addition to the adverse events listed above, the following events have been reported in clinical studies, literature reports, and postmarketing use of products which contain (or are metabolized to) mesalamine. Because many of these events were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to their seriousness or potential causal connection to mesalamine:
Body as a Whole: Neck pain, facial edema, peripheral edema, edema, lupus-like syndrome, drug fever (rare).
Cardiovascular: Pericarditis (rare), myocarditis (rare), vasodilation.
Gastrointestinal: Anorexia, pancreatitis, gastritis, increased appetite, abdominal enlargement, cholecystitis, dry mouth, oral ulcers, perforated peptic ulcer (rare), bloody diarrhea, rectal hemorrhage. There have been rare reports of hepatotoxicity including, jaundice, cholestatic jaundice, hepatitis, and possible hepatocellular damage including liver necrosis and liver failure. Some of these cases were fatal. Asymptomatic elevations of liver enzymes which usually resolve during continued use or with discontinuation of the drug have also been reported. One case of Kawasaki-like syndrome which included changes in liver enzymes was also reported.
Hematologic: Agranulocytosis (rare), aplastic anemia (rare), thrombocytopenia, eosinophilia, leukopenia, anemia, lymphadenopathy.
Musculoskeletal: Gout, arthritis.
Nervous: Depression, somnolence, emotional lability, hyperesthesia, paresthesia, vertigo, confusion, insomnia, migraine, peripheral neuropathy (rare), transverse myelitis (rare), Guillain-Barré syndrome (rare).
Respiratory/Pulmonary: Eosinophilic pneumonia, interstitial pneumonitis, asthma exacerbation, bronchitis, pleuritis, increased cough.
Skin: Alopecia, psoriasis (rare), pyoderma gangrenosum (rare), dry skin, erythema nodosum, urticaria.
Special Senses: Eye pain, taste perversion, blurred vision, tinnitus, ear disorder, ear pain.
Urogenital: Renal Failure (rare), interstitial nephritis, minimal change nephropathy (See also Renal subsection in PRECAUTIONS). Dysuria, urinary urgency, epididymitis, menorrhagia.
Laboratory Abnormalities: Elevated AST (SGOT) or ALT (SGPT), elevated alkaline phosphatase, elevated GGT, elevated LDH, elevated bilirubin, elevated serum creatinine and BUN.
7. DRUG INTERACTIONS
There are no known drug interactions.
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Teratogenic Effects: Pregnancy Category B: Reproduction studies in rats at oral doses up to 480 mg/kg/day (about 1.6 times the recommended human treatment dose on a body surface area basis) and rabbits at oral doses up to 480 mg/kg/day (about 3.2 times the recommended human treatment dose on a body surface area basis) have revealed no evidence of impaired fertility or harm to the fetus due to mesalamine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.2. Labor and Delivery
There are no adequate and well-controlled studies of the use of mesalamine during labor and delivery.
8.3. Nursing Mothers
Low concentrations of mesalamine and higher concentrations of its N-acetyl metabolite have been detected in human breast milk. While the clinical significance of this has not been determined, caution should be exercised when mesalamine is administered to a nursing woman.
8.4. Pediatric Use
Safety and effectiveness of Asacol tablets in pediatric patients have not been established.
8.5. Geriatric Use
Clinical studies of Asacol did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing Asacol. Reports from uncontrolled clinical studies and post-marketing reporting systems suggest a higher incidence of blood dyscrasias, i.e., agranulocytosis, neutropenia, pancytopenia, in subjects receiving Asacol who are 65 years or older. Caution should be taken to closely monitor blood cell counts during drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken when prescribing this drug therapy. As stated in the PRECAUTIONS section, it is recommended that all patients have an evaluation of renal function prior to initiation of Asacol tablets and periodically while on Asacol therapy.
10. OVERDOSAGE
There is no documented clinical experience with mesalamine overdose. There is no specific antidote, and treatment should be symptomatic and supportive.
In dogs, single doses of 6 grams of delayed-release Asacol tablets resulted in renal papillary necrosis but were not fatal. This was approximately 12.5 times the recommended human dose (based on a dose of 2.4 g/day in a 50 kg person). Single oral doses of uncoated mesalamine in mice and rats of 5000 mg/kg and 4595 mg/kg, respectively, or of 3000 mg/kg in cynomolgus monkeys, caused significant lethality.
11. DESCRIPTION
Each Asacol delayed-release tablet for oral administration contains 400 mg of mesalamine, an anti-inflammatory drug. The Asacol delayed-release tablets are coated with acrylic based resin, Eudragit S (methacrylic acid copolymer B, NF), which dissolves at pH 7 or greater, releasing mesalamine in the terminal ileum and beyond for topical anti-inflammatory action in the colon. Mesalamine has the chemical name 5-amino-2-hydroxybenzoic acid; its structural formula is:
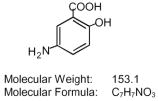
Inactive Ingredients: Each tablet contains colloidal silicon dioxide, dibutyl phthalate, edible black ink, iron oxide red, iron oxide yellow, lactose, magnesium stearate, methacrylic acid copolymer B (Eudragit S), polyethylene glycol, povidone, sodium starch glycolate, and talc.
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mesalamine is thought to be the major therapeutically active part of the sulfasalazine molecule in the treatment of ulcerative colitis. Sulfasalazine is converted to equimolar amounts of sulfapyridine and mesalamine by bacterial action in the colon. The usual oral dose of sulfasalazine for active ulcerative colitis is 3 to 4 grams daily in divided doses, which provides 1.2 to 1.6 grams of mesalamine to the colon.
The mechanism of action of mesalamine (and sulfasalazine) is unknown, but appears to be topical rather than systemic. Mucosal production of arachidonic acid (AA) metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs), is increased in patients with chronic inflammatory bowel disease, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin (PG) production in the colon.
12.3 Pharmacokinetics
Asacol tablets are coated with an acrylic-based resin that delays release of mesalamine until it reaches the terminal ileum and beyond. This has been demonstrated in human studies conducted with radiological and serum markers. Approximately 28% of the mesalamine in Asacol tablets is absorbed after oral ingestion, leaving the remainder available for topical action and excretion in the feces. Absorption of mesalamine is similar in fasted and fed subjects. The absorbed mesalamine is rapidly acetylated in the gut mucosal wall and by the liver. It is excreted mainly by the kidney as N-acetyl-5-aminosalicylic acid.
Mesalamine from orally administered Asacol tablets appears to be more extensively absorbed than the mesalamine released from sulfasalazine. Maximum plasma levels of mesalamine and N-acetyl-5-aminosalicylic acid following multiple Asacol doses are about 1.5 to 2 times higher than those following an equivalent dose of mesalamine in the form of sulfasalazine. Combined mesalamine and N-acetyl-5-aminosalicylic acid AUC's and urine drug dose recoveries following multiple doses of Asacol tablets are about 1.3 to 1.5 times higher than those following an equivalent dose of mesalamine in the form of sulfasalazine.
The tmax for mesalamine and its metabolite, N-acetyl-5-aminosalicylic acid, is usually delayed, reflecting the delayed-release, and ranges from 4 to 12 hours. The half-lives of elimination (t1/2elm) for mesalamine and N-acetyl-5-aminosalicylic acid are usually about 12 hours, but are variable, ranging from 2 to 15 hours. There is a large intersubject variability in the plasma concentrations of mesalamine and N-acetyl-5-aminosalicylic acid and in their elimination half-lives following administration of Asacol tablets.
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dietary mesalamine was not carcinogenic in rats at doses as high as 480 mg/kg/day, or in mice at 2000 mg/kg/day. These doses are 2.4 and 5.1 times the maximum recommended human maintenance dose of Asacol of 1.6 g/day (32 mg/kg/day if 50 kg body weight assumed or 1184 mg/m2), respectively, based on body surface area. Mesalamine was negative in the Ames assay for mutagenesis, negative for induction of sister chromatid exchanges (SCE) and chromosomal aberrations in Chinese hamster ovary cells in vitro, and negative for induction of micronuclei (MN) in mouse bone marrow polychromatic erythrocytes. Mesalamine, at oral doses up to 480 mg/kg/day (about 1.6 times the recommended human treatment dose on a body surface area basis), was found to have no effect on fertility or reproductive performance of male and female rats.
Study to assess the effect on male fertility: The effect of Asacol (mesalamine) on sulfasalazine-induced impairment of male fertility was examined in an open-label study. Nine patients (age < 40 years) with chronic ulcerative colitis in clinical remission on sulfasalazine 2 g/day to 3 g/day were crossed over to an equivalent Asacol dose (0.8 g/day to 1.2 g/day) for 3 months. Improvement in sperm count (p < 0.02) and morphology (p < 0.02) occurred in all cases. Improvement in sperm motility (p < 0.001) occurred in 8 of the 9 patients.
13.2 Animal Toxicology and/or Pharmacology
See 5.1 Renal Warnings and Precautions
14. CLINICAL STUDIES
14.1 Mildly to moderately active ulcerative colitis:
Two placebo-controlled studies have demonstrated the efficacy of Asacol tablets in patients with mildly to moderately active ulcerative colitis. In one randomized, double-blind, multi-center trial of 155 patients, Asacol doses of 1.6 g/day and 2.4 g/day were compared to placebo. At the dose of 2.4 g/day, Asacol tablets reduced the disease activity, with 21 of 43 (49%) Asacol patients showing improvement in sigmoidoscopic appearance of the bowel compared to 12 of 44 (27%) placebo patients (p = 0.048). In addition, significantly more patients in the Asacol 2.4 g/day group showed improvement in rectal bleeding and stool frequency. The 1.6 g/day dose did not produce consistent evidence of effectiveness.
In a second randomized, double-blind, placebo-controlled clinical trial of 6 weeks duration in 87 ulcerative colitis patients, Asacol tablets, at a dose of 4.8 g/day, gave sigmoidoscopic improvement in 28 of 38 (74%) patients compared to 10 of 38 (26%) placebo patients (p < 0.001). Also, more patients in the Asacol 4.8 g/day group showed improvement in overall symptoms.
14.2 Maintenance of remission of ulcerative colitis:
A 6-month, randomized, double-blind, placebo-controlled, multi-center study involved 264 patients treated with Asacol 0.8 g/day (n = 90), 1.6 g/day (n = 87), or placebo (n = 87). The proportion of patients treated with 0.8 g/day who maintained endoscopic remission was not statistically significant compared to placebo. In the intention to treat (ITT) analysis of all 174 patients treated with Asacol 1.6 g/day or placebo, Asacol maintained endoscopic remission of ulcerative colitis in 61 of 87 (70.1%) of patients, compared to 42 of 87 (48.3%) of placebo recipients (p = 0.005).
A pooled efficacy analysis of 4 maintenance trials compared Asacol, at doses of 0.8 g/day to 2.8 g/day, with sulfasalazine, at doses of 2 g/day to 4 g/day (n = 200). Treatment success was 59 of 98 (59%) for Asacol and 70 of 102 (69%) for sulfasalazine, a non-significant difference.
16. HOW SUPPLIED/STORAGE AND HANDLING
Asacol tablets are available as red-brown, capsule-shaped tablets containing 400 mg mesalamine and imprinted “Asacol NE” in black.
NDC 0149-0752-15 Bottle of 180
Store at controlled room temperature 20°- 25°C (68°- 77°F) [See USP].
17. PATIENT COUNSELING INFORMATION
Patients should be instructed to swallow the Asacol tablets whole, taking care not to break the outer coating. The outer coating is designed to remain intact to protect the active ingredient and thus ensure mesalamine availability for action in the colon. In 2% to 3% of patients in clinical studies, intact or partially intact tablets have been reported in the stool. If this occurs repeatedly, patients should contact their physician.
Patients with ulcerative colitis should be made aware that ulcerative colitis rarely remits completely, and that the risk of relapse can be substantially reduced by continued administration of Asacol at a maintenance dosage.
Procter & Gamble Pharmaceuticals
Cincinnati, OH 45202
under license from Medeva Pharma Suisse SA
registered trademark owner.
Made in Germany, D-64331 Weiterstadt
U.S. Patent Nos. 5,541,170 and 5,541,171
| Asacol (mesalamine) | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
Revised: 03/2008Procter & Gamble Pharmaceuticals