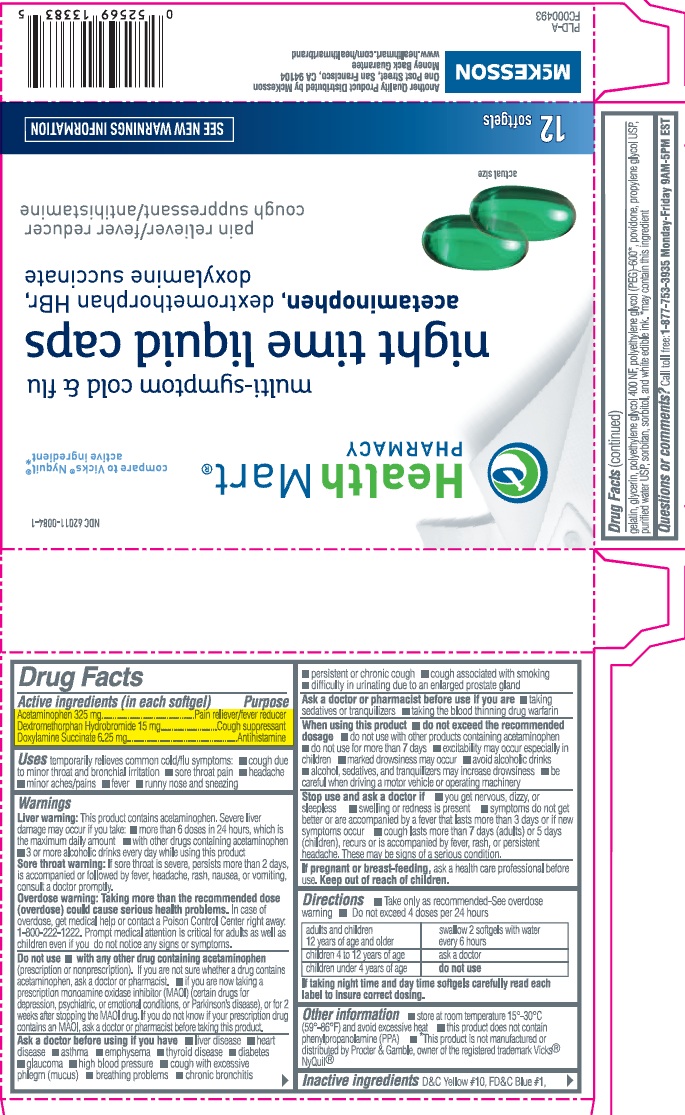
HEALTH MART MULTI-SYMPTOM COLD AND FLU NIGHT TIME - acetaminophen, dextromethorphan hydrobromide and doxylamine succinate capsule, liquid filled
McKesson
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients (in each softgel)
Acetaminophen 325mg
Dextromethorphan Hydrobromide 15mg
Doxylamine Succinate 6.25 mg
Uses
temporarily relieves common cold/flu symptoms
- cough due to minor throat and bronchial irritation
- sore throat pain
- headache
- minor aches/pains
- fever
- runny nose and sneezing
Warnings
Liver warning: This product contains acetaminophen. Sever liver damage may occur if you take:
- more than 6 doses in 24 hours, which is the maxiumum daily amount.
- with other drugs containing acetaminophen,
- 3 or more alcoholic drinks everyday while using this product.
Overdose warning: Taking more than the recommended dose (overdose) could cause serious health problems.
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as children even if you do not notice any signs or symptoms.
Do not use
with any other drug containing acetaminophen (prescription or non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stoppig the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor of pharmacist before taking this product.
Ask a doctor before using if you have
- liver disease
- heart disease
- asthma
- emphysema
- thyroid disease
- diabetes
- glaucoma
- high blood pressure
- cough with excessive phlegm (mucus)
- breathing problems
- chronic bronchitis
- persistent or chronic cough
- cough associated with smoking
- difficulty in urinating due to an enlarged prostate gland
Ask a doctor of pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not exceed the recommended dosage
- do not use with other products containing acetaminophen
- do not use for more than 7 days
- excitability may occur especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives,a nd tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery.
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- swelling or redness is present
- symptoms do not get better or are accompanied by a fever that lasts more than 7 days (adults) or 5 days (children), recurs or is accompanied by fever, rash, or persistent headache. These may be signs of a serious condition.
Directions
- Take only as recommended - See overdose warning
- Do not exceed 4 doses per 24 hours
children 4 to 12 years of age ask a doctor
children under 4 years of age do not use
If taking NiteTime and DayTime softgels carefully read each label to insure correct dosing.
Other Information
store at room temperature 15-30 degrees C (59-86F) and avoid excessive heat.
This product does not contain phenylpropanolamine (PPAP
*This product is not manufactured or distributed by Procter and Gamble, owner of the registered trademear Vicks (r) NyQuil(r)
Distributed by:
PL Developments
Westbury, NY 11590 USA
Questions or Comments?
Call toll free 1-800-753-3935
Keep outer carton for complete warnings and product information.
THIS PRODUCT IS PACKAGED IN A CHILD RESISTANT AND TAMPER EVIDENT PACKAGE. USE ONLY IF BLISTERS ARE INTACT.
*Compare to the active ingredients in Vicks Nyquil
NDC 59726-480-10
SEE NEW WARNINGS INFORMATION
Multi-symptom
NiteTime Liquid Capsules
Cold/Flue Relief
Pain reliever, fever reducer, cough suppressant, antihistimine
PARENTS: learn about teen medicine abuse www.stopmedicineabuse.org
ACETAMINOPHEN
Dextromethorphan HBr, Doxylamine Succinate
10 SOFTGELS
| HEALTH MART MULTI-SYMPTOM COLD AND FLU NIGHT TIME
acetaminophen, dextromethorphan hydrobromide, doxylamine succinate capsule, liquid filled |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - McKesson (177667227) |