MALLY PERFECT PREP PORELESS PRIMER
-
avobenzone and
octinoxate cream
MallyGirl, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
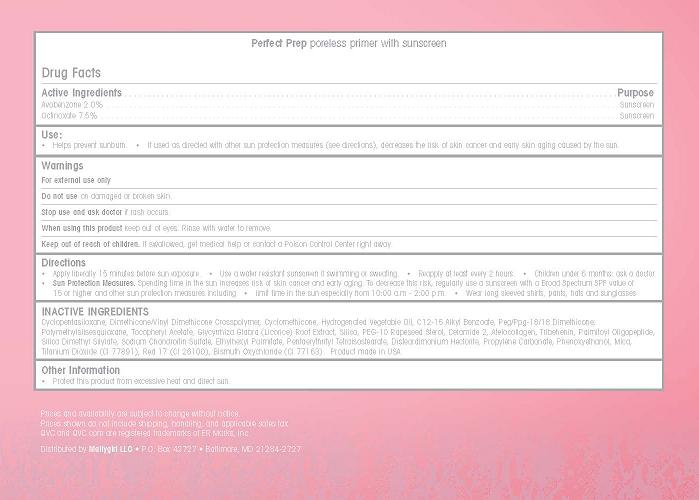
Use
- Helps prevent sunburn
- If used as directed with other sun protection measures (see directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children. If swollowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: ask a doctor
- Sun Protection Measures. Spending time in the sun increases risk of skin cancer and early aging. To decrease this risk regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higer and other sun protection measures including
- Limit time in the sun especially from 10:00 a.m. - 2:00 p.m.
- Wear long sleeved shirts, pants, hats and sunglasses
Inactive Ingredients
Cyclopentasiloxane, Dimethicone/Vinyl Dimeticone Crosspolymer, Cyclomethicone, Hydrogenated Vegetable Oil, C12-15 Alkyl Benzoate, Peg/Ppg-18/18 Dimethicone, Polymethylsilsesquioxane, Tocopheryl Acetate, Glycyrrhiza Glabra (Licorice) Root Extract, Silica, PEG-10 Rapeseed Sterol, Ceramide 2, Atelocollagen, Tribehenin, Palmitoyl Oligopeptide, Silica Dimethyl Silylate, Sodium Chondroitin Sulfate, Pentaerythrityl Tetraisostearate, Ethylthexyl Palmitate, Disteardimonium Hectorite, Propylene Carbonate, Phenoxyethanol, Mica, Titanium Dioxide (CI 77891), Red 17 (CI 26100), Bismuth Oxychloride (CI 77163). Product made in the USA
Prices and availability are subject to change without notice.
Prices shown do not include shipping, handling, and applicable sales tax.
QVC and QVC.com are registered trademarks of ER Marks, Inc.
Distributed by Mallygirl LLC - P.O. Box 42727 - Baltimore, MD 21284-2727
| MALLY PERFECT PREP PORELESS PRIMER
avobenzone, octinoxate cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part352 | 03/29/2012 | |
| Labeler - MallyGirl, LLC (167262554) |
Revised: 03/2012 MallyGirl, LLC