BENZASHAVE
-
benzoyl peroxide cream
E. FOUGERA & CO., A division of Fougera Pharmaceuticals Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION:
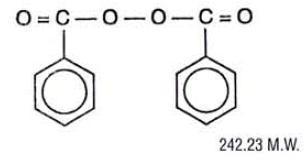
BenzaShave® 5% and BenzaShave® 10% (benzoyl peroxide 5% and 10%) are topical shaving cream preparations for use in the treatment of pseudofolliculitis (p. barbae; ingrown hairs, razor bumps) and acne vulgaris associated with shaving. Benzoyl peroxide is an oxidizing agent which possesses antibacterial properties and is classified as a keratolytic agent. Benzoyl peroxide (C14H10O4) is represented by the following chemical structure.
BenzaShave® 5%
Each gram of Benzashave® (benzoyl peroxide 5%) contains: benzoyl peroxide 50mg, purified water, edetate disodium, propylparaben, methylparaben, carbomer interpolymer A, sodium coco-sulfate, sodium lauroamphoacetate, glycerin, stearic acid, myristic acid, dimethicone copolyol, mineral oil and coconut acid.
BenzaShave® 10%
Each gram of Benzashave® (benzoyl peroxide 10%) contains: benzoyl peroxide 100mg, purified water, edetate disodium, propylparaben, methylparaben, carbomer interpolymer A, sodium coco-sulfate, sodium lauroamphoacetate, glycerin, stearic acid, myristic acid, dimethicone copolyol, mineral oil and coconut acid.
CLINICAL PHARMACOLOGY:
The mechanism of action of benzoyl peroxide has not been determined but may be related to its antibacterial activity against Propionibacterium acnes and its ability to cause drying and peeling. Benzoyl peroxide reduces the concentration of free fatty acids in the sebum. Little is known about the percutaneous penetration, metabolism and excretion of benzoyl peroxide, although it is likely that benzoic acid is a major metabolite. There is no evidence of systemic toxicity caused by benzoyl peroxide in humans.
INDICATIONS AND USAGE:
A medicated shaving cream containing 5% or 10% benzoyl peroxide that effectively intervenes to aid in the treatment of pseudofolliculitis (p. barbae, ingrown hairs, razor bumps) and acne vulgaris associated with shaving. This formula primes and lifts hair follicles, promoting a clean, close shave.
CONTRAINDICATIONS:
These products are contraindicated in patients with a history of hypersensitivity to benzoyl peroxide or to any of the other ingredients of the preparations.
PRECAUTIONS:
General: For external use only. This preparation should not be used in or near the eyes or on mucous membranes. If severe irritation develops, discontinue use and institute appropriate therapy. After the reaction clears, treatment may often be resumed with less frequent application.
Information for Patients: Avoide contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. If excessive irritation develops, discontinue use and consult a physician. May bleach hair and colored fabric.
Carcinogenesis, Mutagenesis, and Impairment of Fertility: Data from several studies using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of these findings to humans is unknown.
Pregnancy Category C: Animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed. There are no data available on the effect of benzoyl peroxide on the growth, development and functional maturation of the unborn child.
ADVERSE REACTIONS:
Allergic contact dermatitis has been reported with topical benzoyl peroxide therapy.
DOSAGE AND ADMINISTRATION:
Wet area to be shaved. Apply a small amount of Benzashave® with fingertips. Gently rub over entire area and shave.
HOW SUPPLIED:
Benzashave® (benzoyl peroxide 5%), 4 oz (113.4 g) tube, NDC 10337-805-41.
Benzashave® (benzoyl peroxide 10%), 4 oz (113.4 g) tube, NDC 10337-806-41.
KEEP OUT OF REACH OF CHILDREN.
Store at Controlled Room Temperature 15°-25°C (59°-77°F)
FOR EXTERNAL USE ONLY.
NOT FOR OPHTHALMIC USE.
For control number and expiration date, see crimp of tube.
Manufacture for:
DERMarts® Division
DOAK DERMATOLOGICS
A Subsidiary of Bradley Pharmaceuticals, Inc.
383 Route 46 West
Fairfield, NJ 07004-2402 USA
www.doakderm.com
Manufactured by:
Groupe PARIMA, Inc.
Montreal, QC, H4S 1X6 CANADA
IN-IL249
ISS: 08/05
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 113.4 G CONTAINER
NDC 10337-805-41
Rx Only
BENZASHAVE ® 5%
(benzoyl peroxide 5%)
Medicated
Shaving Cream
DOAK DERMATOLOGICS
Net Wt. 4oz (113.4 g)
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 113.4 G CARTON
NDC 10337-805-41
Rx Only
BENZA SHAVE® 5%
(benzoyl peroxide 5%)
Medicated
Shaving Cream
DOAK DERMATOLOGICS
Net Wt. 4oz (113.4g)
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 113.4 G CONTAINER
NDC 10337-806-41
Rx Only
BENZASHAVE ® 10%
(benzoyl peroxide 10%)
Medicated
Shaving Cream
DOAK DERMATOLOGICS
Net Wt. 4oz (113.4 g)
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 113.4 G CARTON
NDC 10337-806-41
Rx Only
BENZASHAVE® 10%
(benzoyl peroxide 10%)
Medicated
Shaving Cream
DOAK DERMATOLOGICS
Net Wt. 4oz (113.4g)
| BENZASHAVE
benzoyl peroxide cream |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 11/01/2005 | ||
| BENZASHAVE
benzoyl peroxide cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 03/30/2012 | ||
| Labeler - E. FOUGERA & CO., A division of Fougera Pharmaceuticals Inc. (043838424) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Fougera Pharmaceuticals Inc. | 043838424 | ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Groupe PARIMA Inc. | 252437850 | manufacture | |
Revised: 04/2012 E. FOUGERA & CO., A division of Fougera Pharmaceuticals Inc.



