MECLIZINE HYDROCHLORIDE
-
meclizine hydrochloride tablet
Sandoz Inc
----------
DESCRIPTION
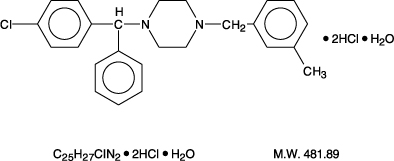
Meclizine hydrochloride, an oral antiemetic, is a white, slightly yellowish, crystalline powder which has a slight odor and is tasteless. Chemically, meclizine HCl is 1-(p-chloro-α-phenylbenzyl)-4-m-methylbenzyl) piperazine dihydrochloride monohydrate.
Each tablet for oral administration contains 12.5 mg or 25 mg meclizine hydrochloride. Inactive ingredients include: croscarmellose sodium, hydroxypropyl methylcellulose, lactose (monohydrate), magnesium stearate, microcrystalline cellulose, polysorbate 80. The 12.5 mg also contains FD & C Blue #1 Aluminum Lake; the 25 mg also contains D & C Yellow #10 Aluminum Lake and FD & C Yellow #6 Aluminum Lake.
CLINICAL PHARMACOLOGY
Meclizine HCl is an antihistamine which shows marked protective activity against nebulized histamine and lethal doses of intravenously injected histamine in guinea pigs. It has a marked effect in blocking the vasodepressor response to histamine, but only a slight blocking action against acetylcholine. Its activity is relatively weak in inhibiting the spasmogenic action of histamine on isolated guinea pig ileum.
INDICATIONS AND USAGE
For the prevention and treatment of nausea, vomiting or dizziness associated with motion sickness.
CONTRAINDICATIONS
Meclizine HCl is contraindicated in individuals who have shown a previous hypersensitivity to it.
WARNINGS
Since drowsiness may, on occasion, occur with use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery.
Patients should avoid alcoholic beverages while taking this drug. Due to its potential anticholinergic action, this drug should be used with caution in patients with asthma, glaucoma, or enlargement of the prostate gland.
Do not give to children under 12 years of age unless directed by a doctor.
PRECAUTIONS
Pregnancy, Teratogenic Effects, Pregnancy Category B
Reproduction studies in rats have shown cleft palates at 25-50 times the human dose. Epidemiological studies in pregnant women, however, do not indicate that meclizine increases the risk of abnormalities when administered during pregnancy. Despite the animal findings, it would appear that the possibility of fetal harm is remote. Nevertheless, meclizine, or any other medication, should be used during pregnancy only if clearly necessary.
HOW SUPPLIED
Meclizine HCl tablets for oral administration are available as:
12.5 mg: Oval blue scored tablets, debossed GG 141 on one side and plain on the reverse side and supplied as:
NDC 0781-1542-01 bottles of 100
NDC 0781-1542-10 bottles of 1000
25 mg: Oval yellow scored tablets, debossed GG 261 on one side and plain on the reverse side and supplied as:
NDC 0781-1544-01 bottles of 100
NDC 0781-1544-10 bottles of 1000
| MECLIZINE HYDROCHLORIDE
meclizine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA084843 | 05/22/1989 | 08/31/2010 |
| MECLIZINE HYDROCHLORIDE
meclizine hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA084092 | 05/22/1989 | 09/30/2010 |
| Labeler - Sandoz Inc (110342024) |
Revised: 02/2012 Sandoz Inc