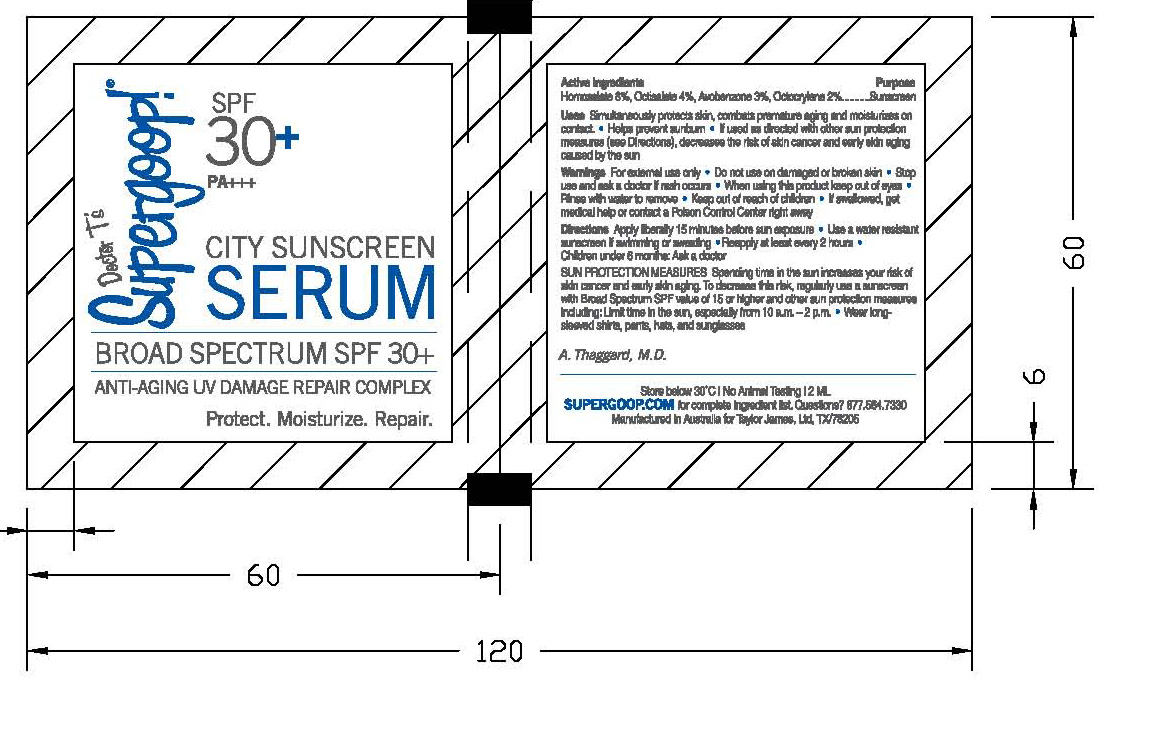
CITY SUNSCREEN SERUM- homosalate, octisalate, avobenzone and octocrylene cream
Taylor James, Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients: Purpose
Homosalate 8%, Octisalate 4%, Avobenzene 3%, Octocrylene 2% Sunscreen
Uses A light face and neck serum with three fold functions to protect skin from UVA and UVB sun exposure, stimulate repair mechanisms, and provide 24 hour moisture simultaneously. Clinical Tests with uniprotect PT-3 show significantly sings of aging including wrinkles and age spot formations.
For external use only. Keep out of of eyes. Rinse with water to remove. Overexposure to the sun is a serious health threat.
Directions Apply 1-2 pumps to face and neck each morning each morning. Massage into skin and reapply after swimming or water exposure. Can be used alone or under makeup as a primer.
Inactive Ingredients Purified water (Aqua), Cyclometicone, Isostearyl Neopentanoate, Glycerin, Ceteareth-20, Polypropylene, Cetearyl Alcohol, Xanthan Gum, d-Panthenol, Octanohydroxamic acid, Caprylyl Glycol, Silica, Triacontanyl PVP, Cetyl Dimethicone, Ammonium Acryloyldimethyltaurate/VP Copolymer, PEG-40 Stearate, Tocopheryl, Disodium EDTA, Pentylene Glycol, Pantheyl Triacetate, Sodium Lactate, Lactic Acid, Serine, Urea, Sorbitol, Sodium Chloride, Allantoin, Oleyl Alcohol, Ethyl Linoleate.


| CITY SUNSCREEN
SERUM
homosalate, octisalate, avobenzene, octocrylene cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Taylor James, Ltd. (033381850) |
| Registrant - Baxter Laboratories Pty. Ltd. (740537709) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Baxter Laboratories Pty. Ltd. | 740537709 | manufacture | |
