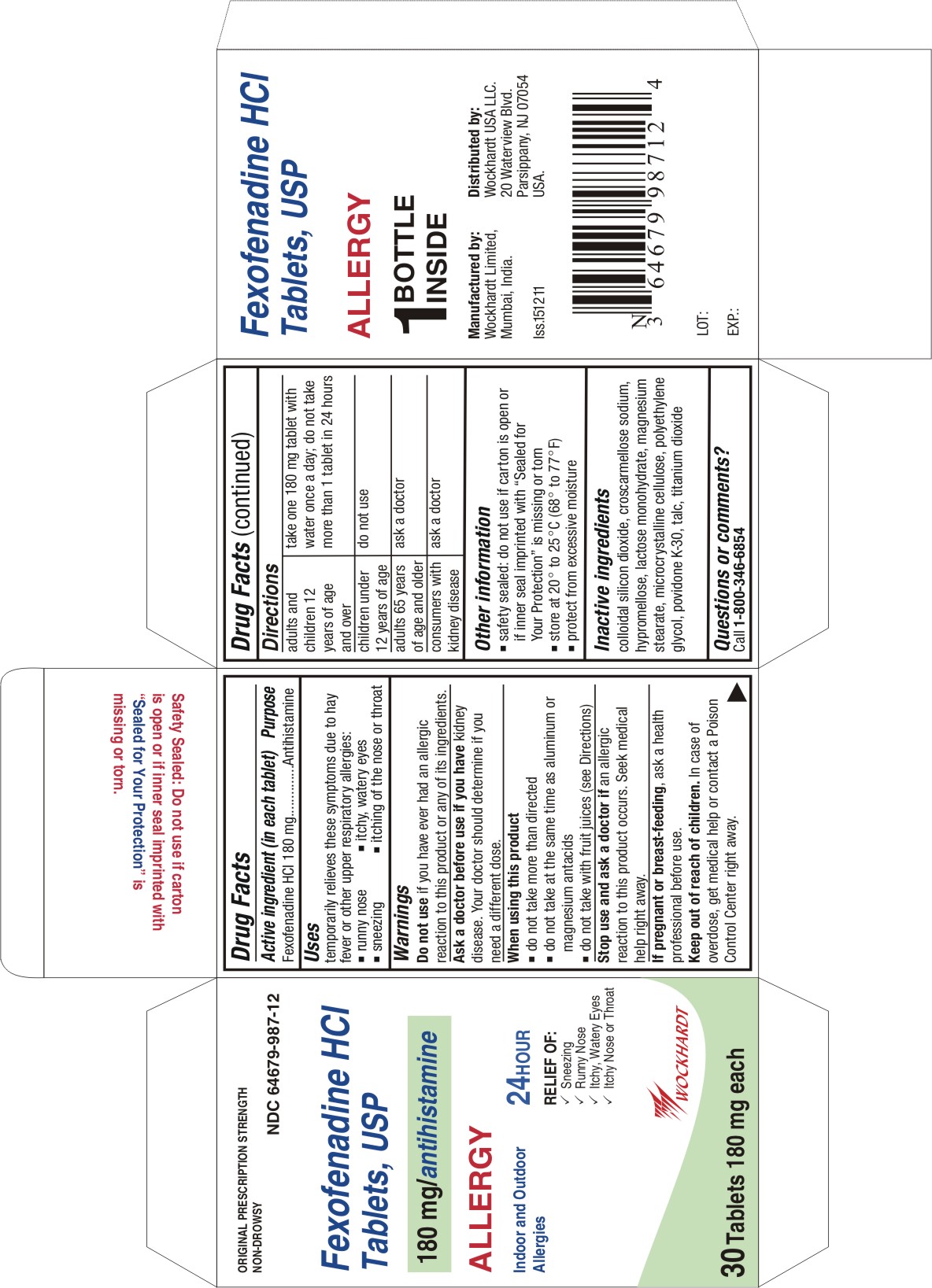
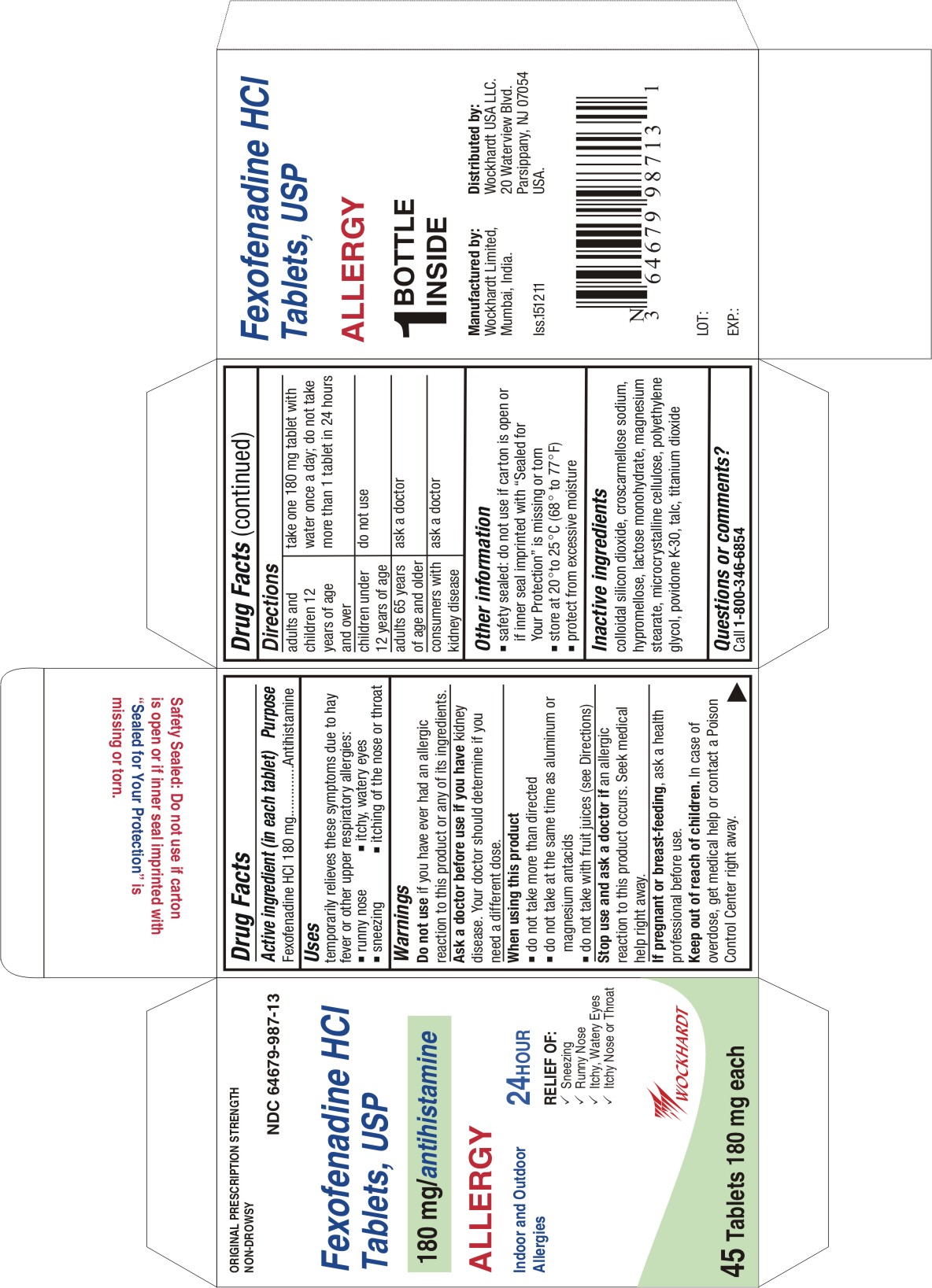
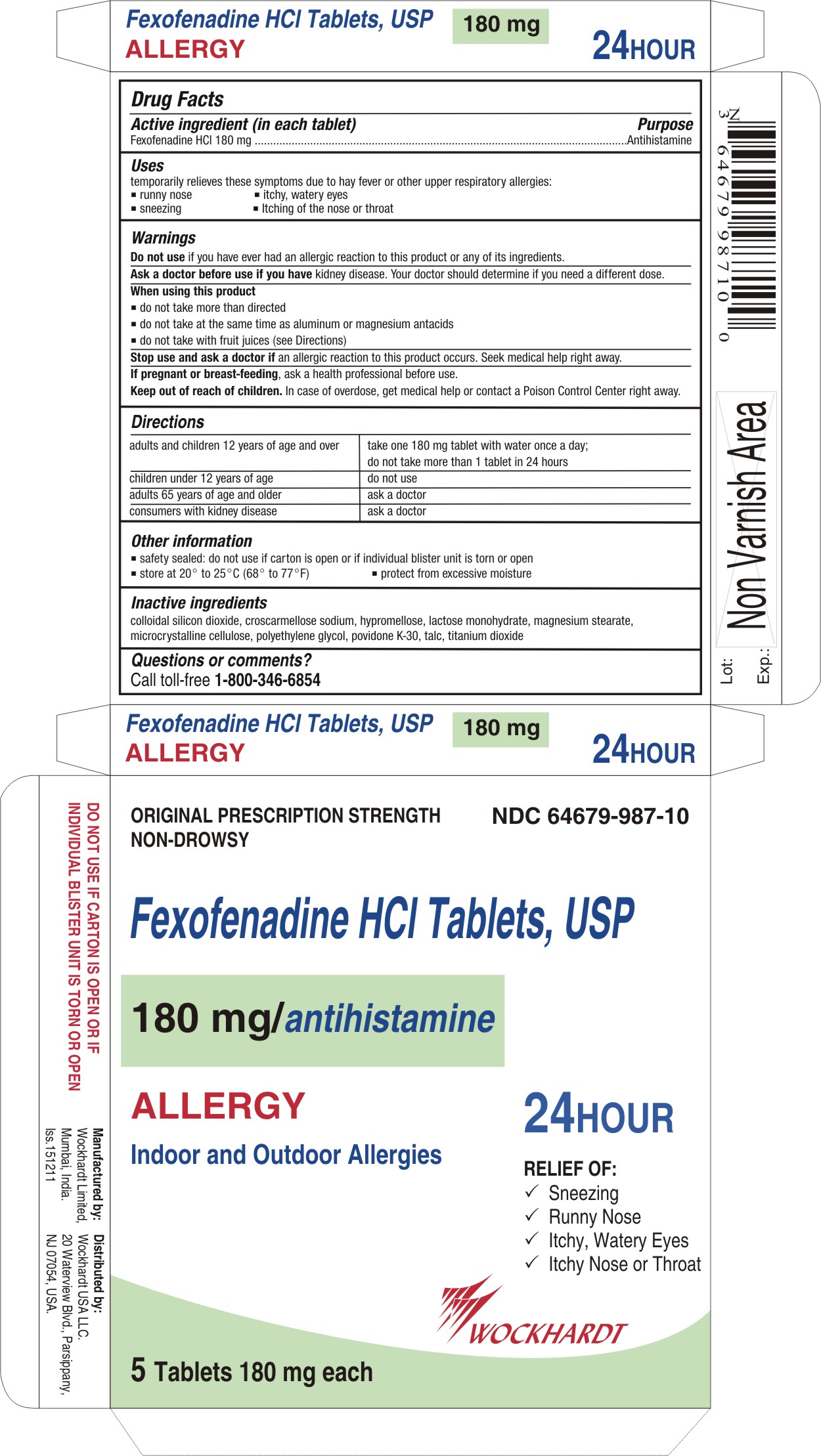
FEXOFENADINE
-
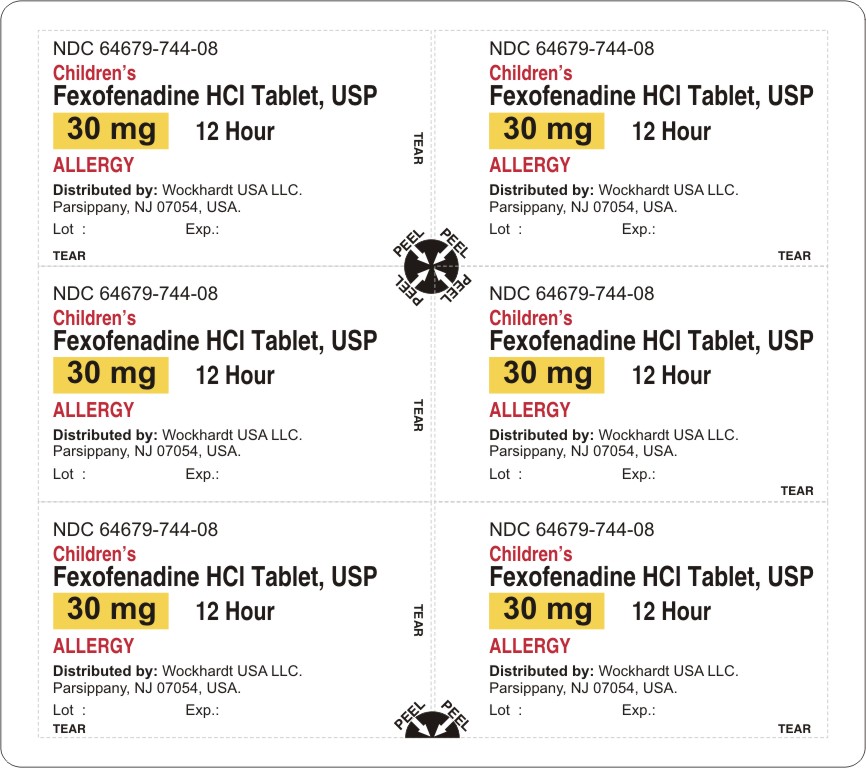
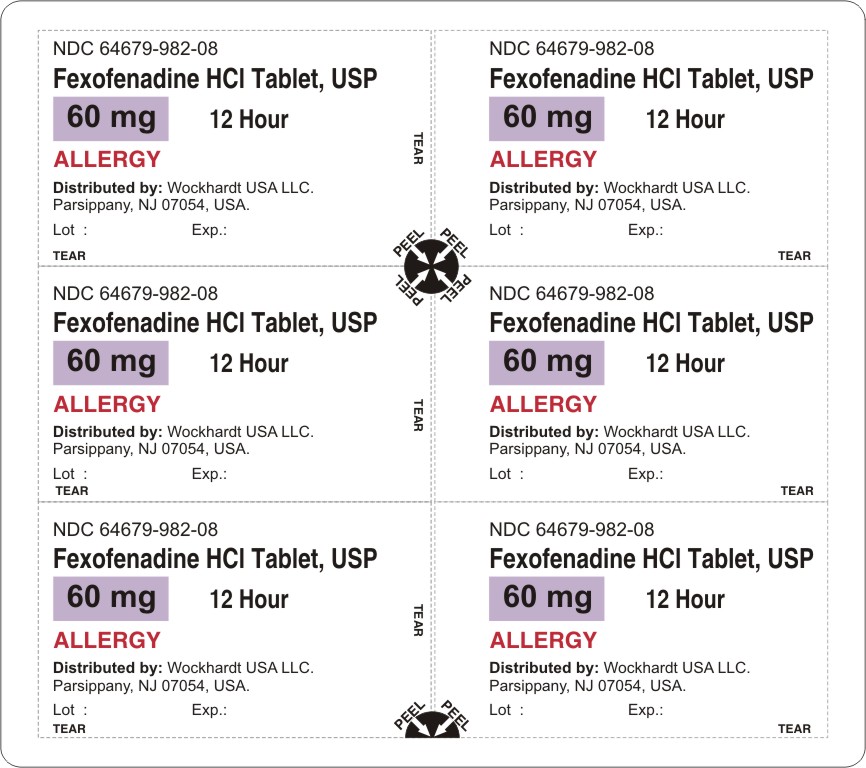
fexofenadine hydrochloride tablet, film coated
Wockhardt USA LLC.
----------
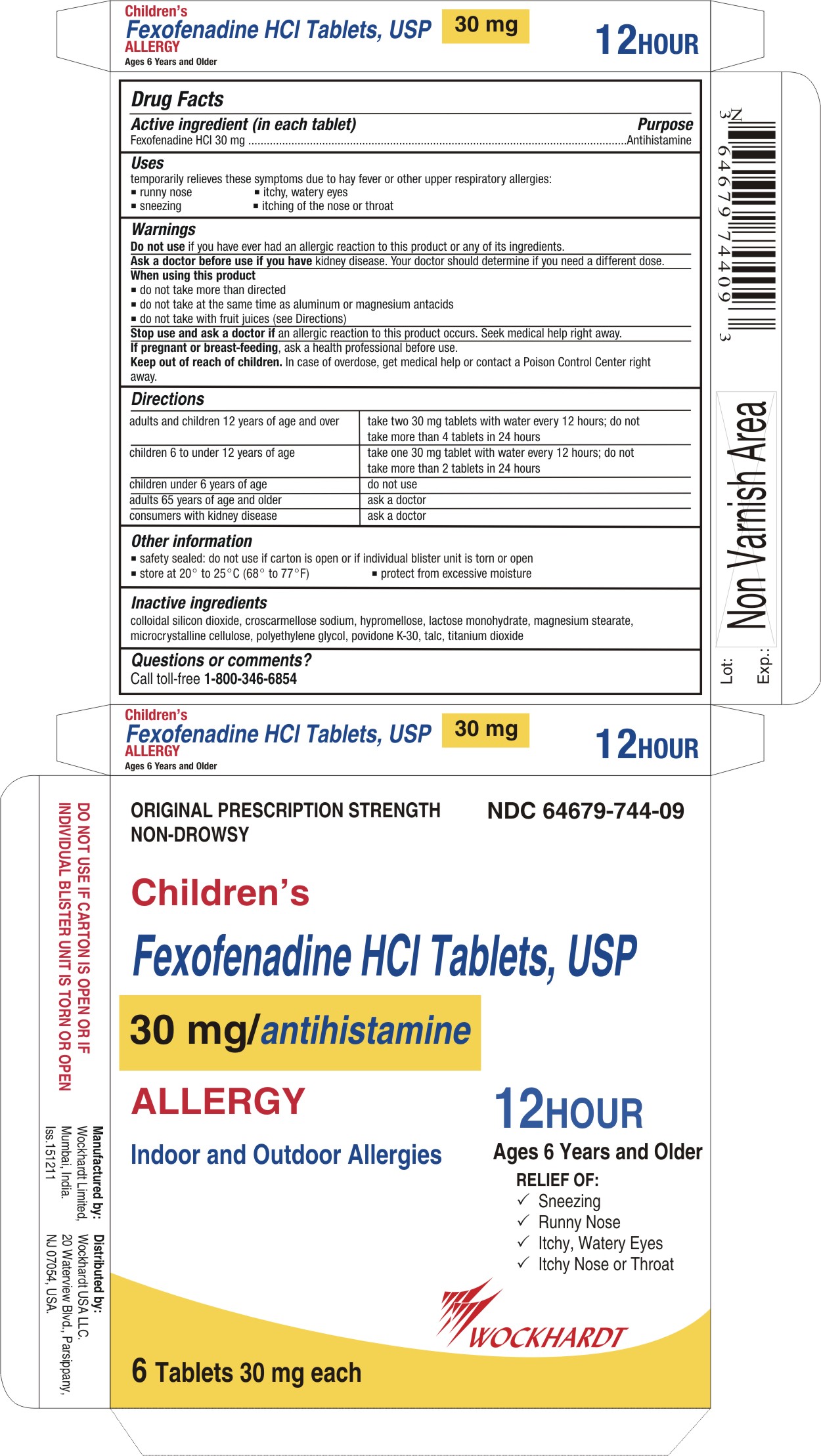
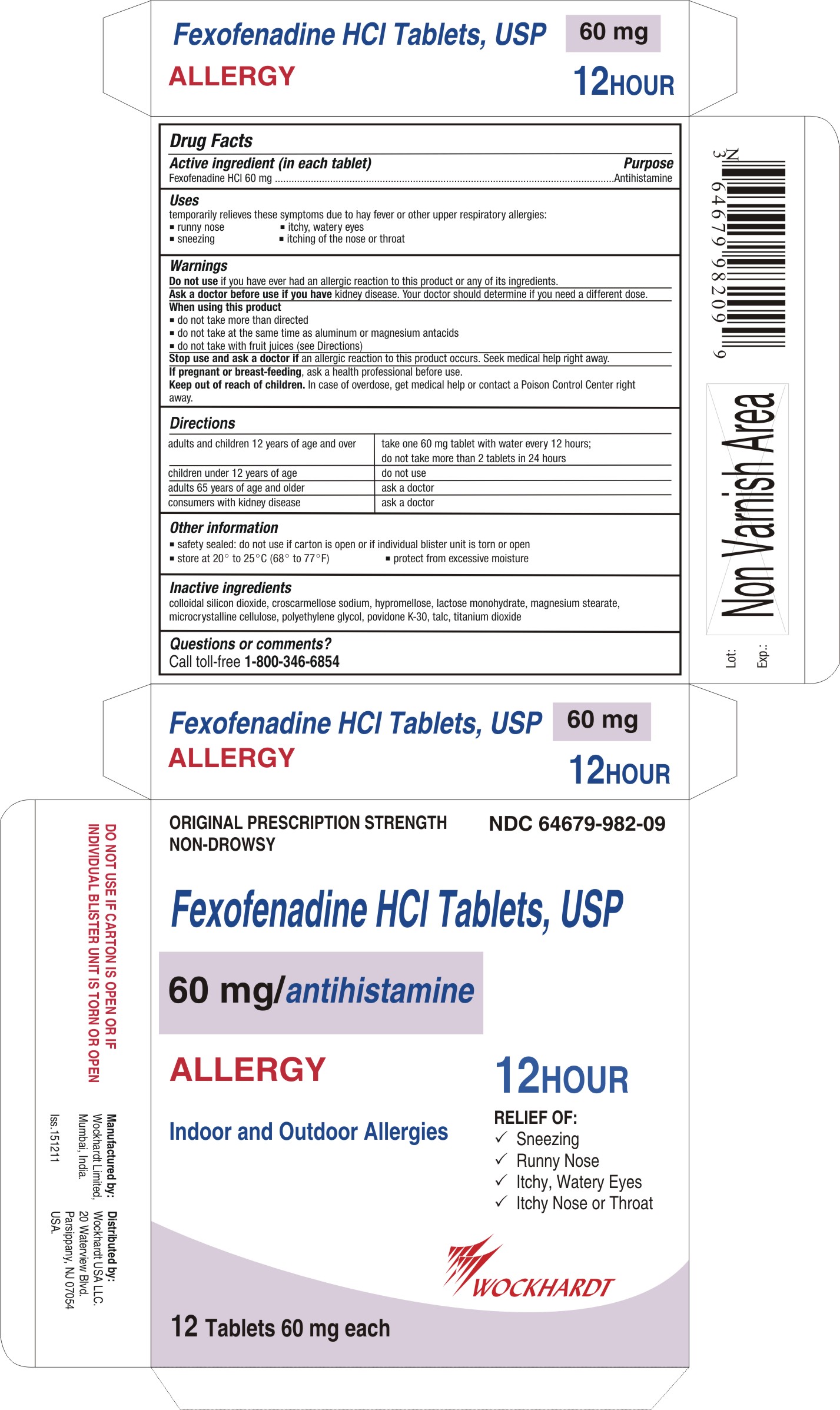
Active ingredient (in each tablet)
For 30 mg:
Fexofenadine HCl 30 mg
For 60 mg:
Fexofenadine HCl 60 mg
For 180 mg:
Fexofenadine HCl 180 mg
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding
ask a health professional before use.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
For 30 mg:
adults and children 12 years of age and over
| take two 30 mg tablets with water every 12 hours;
do not take more than 4 tablets in 24 hours
|
children 6 to under 12 years of age
| take one 30 mg tablet with water every 12 hours;
do not take more than 2 tablets in 24 hours
|
children under 6 years of age
| do not use
|
adults 65 years of age and older
| ask a doctor
|
consumers with kidney disease
| ask a doctor
|
For 60 mg:
adults and children 12 years of age and over
| take one 60 mg tablet with water every 12 hours;
do not take more than 2 tablets in 24 hours
|
children under 12 years of age
| do not use
|
adults 65 years of age and older
| ask a doctor
|
consumers with kidney disease
| ask a doctor
|
For 180 mg:
adults and children 12 years of age and over
| take one 180 mg tablet with water once a day;
do not take more than 1 tablet in 24 hours
|
children under 12 years of age
| do not use
|
adults 65 years of age and older
| ask a doctor
|
consumers with kidney disease
| ask a doctor
|
Other information
- safety sealed: do not use if carton is open or if inner seal imprinted with "Sealed for Your Protection" is missing or torn
- safety sealed: do not use if carton is open or if individual blister unit is torn or open
- store between 20° to 25°C (68° to 77°F)
- protect from excessive moisture
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone K-30, talc, titanium dioxide
Questions or comments?
Call toll-free 1-800-346-6854
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FEXOFENADINE
fexofenadine tablet, film coated |
|
|
|
|
|
|
|
|
|
|
FEXOFENADINE
fexofenadine tablet, film coated |
|
|
|
|
|
|
|
|
|
|
FEXOFENADINE
fexofenadine tablet, film coated |
|
|
|
|
|
|
|
|
|
|
Revised: 02/2012 Wockhardt USA LLC.