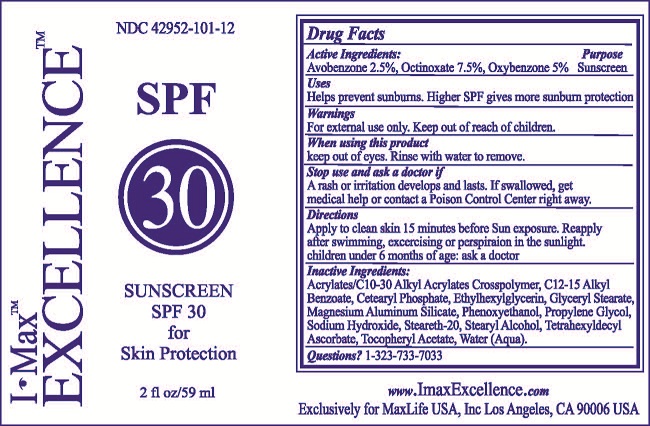
I-MAX EXCELLENCE
-
avobenzone,
octinoxate and
oxybenzone lotion
MAXLIFE USA, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
STOP USE AND ASK A DOCTOR IF
A RASH OR IRRITATION DEVELOPS AND LASTS. IF SWALLOWED, GET
MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS
APPLY TO CLEAN SKIN 15 MINUTES BEFORE SUN EXPOSURE. REAPPLY
AFTER SWIMMING, EXCERCISING OR PERSPIRATION IN THE SUNLIGHT.
CHILDREN UNDER 6 MONTHS OF AGE: ASK A DOCTOR.
INACTIVE INGREDIENTS:
ALKYL BENZOATE, ACRYLATES/C10-30 ALKYL ACRYLATES CROSSPOLYMER, C12-15 GLYCERYL STEARATE, CETEARYL PHOSPHATE, ETHYLHEXYLGLYCERIN, MAGNESIUM ALUMINUM SILICATE, PHENOXYETHANOL, PROPYLENE GLYCOL, SODIUM HYDROXIDE, STEARETH-20, STEARYL ALCOHOL, TETRAHEXYLDECYL ASCORBATE, TOCOPHERYL ACETATE, WATER (AQUA).
| I-MAX EXCELLENCE
avobenzone octinoxate oxybenzone lotion |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part352 | 03/15/2012 | |
| Labeler - MAXLIFE USA, INC. (785111431) |
| Registrant - MAXLIFE USA, INC. (785111431) |
Revised: 04/2012 MAXLIFE USA, INC.