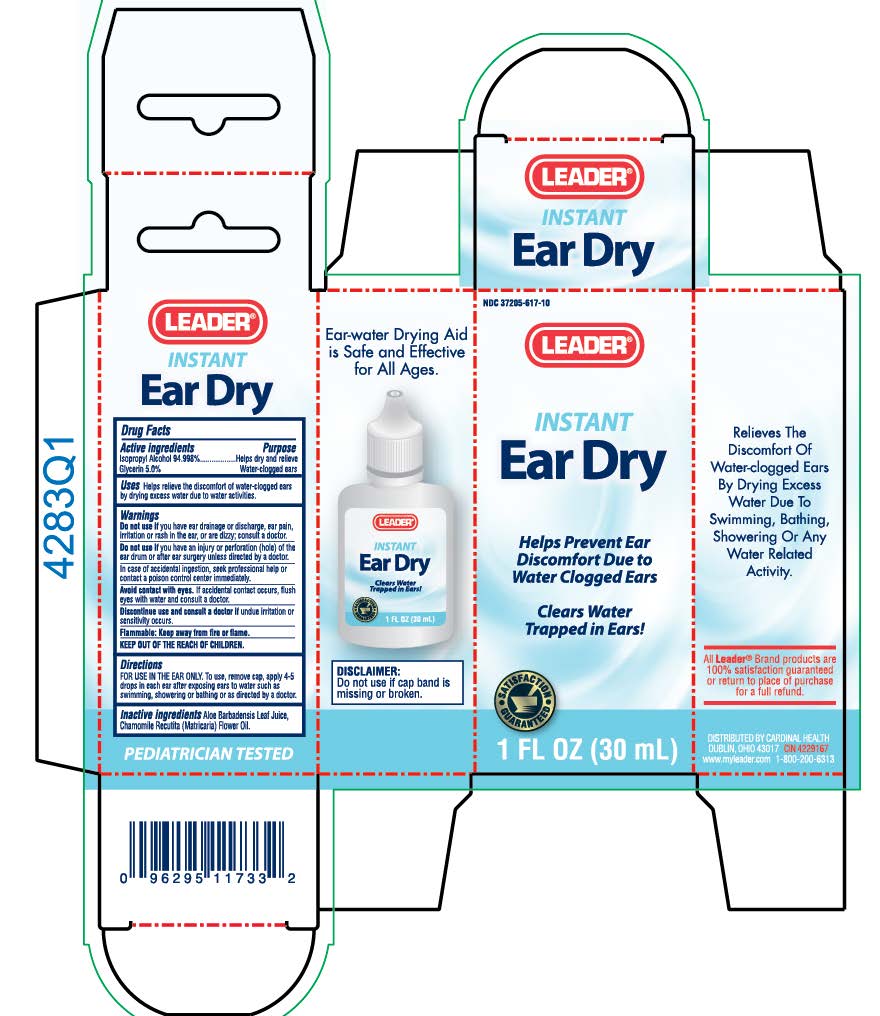
EAR DRY LEADER
-
isopropyl alcohol and
glycerin liquid
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
Helps relieves the discomfort of water-clogged ears by drying excess water due to water activities.
Warnings
Do not use: if you have ear drainage or discharge, ear pain, irritation or rash in the ear, or are dizzy; consult a doctor. If you have an injury or perforation (hole) of the ear drum of after ear surgery unless directed by a doctor. Avoid contact with eyes. If accidental contact occurs, flush eyes with water and consult a doctor. Discontinue use and consult a doctor if: undue irritation or sensitivity occurs.
Directions
FOR USE IN THE EAR ONLY. To use: remove cap, apply 4-5 drops in each ear after exposing ears to water such as swimming, showering or bathing or as directed by a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
| EAR DRY
LEADER
isopropyl alcohol, glycerin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part344 | 12/01/2011 | |
| Labeler - Cardinal Health (097537435) |
| Registrant - Product Quest Mfg, LLC (927768135) |
Revised: 01/2011 Cardinal Health