multihance (gadobenate dimeglumine) injection, solution
[Bracco Diagnostics Inc.]
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with:
- acute or chronic severe renal insufficiency (glomerular filtration rate <30 mL/min/1.73m2), or
- acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period.
In these patients, avoid use of gadolinium-based contrast agents unless the diagnostic information is essential and not available with non-contrast enhanced magnetic resonance imaging (MRI). NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs. Screen all patients for renal dysfunction by obtaining a history and/or laboratory tests. When administering a gadolinium-based contrast agent, do not exceed the recommended dose and allow a sufficient period of time for elimination of the agent from the body prior to any readministration (See WARNINGS).
DESCRIPTION
MULTIHANCE injection is supplied as a sterile, non-pyrogenic, clear, colorless aqueous solution intended for intravenous use only. Each mL of solution contains 529 mg gadobenate dimeglumine. MULTIHANCE contains no preservatives.
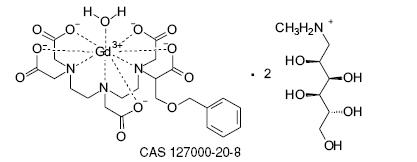
Gadobenate dimeglumine is chemically designated as (4RS)-[4-carboxy-5,8,11-tris(carboxymethyl)-1-phenyl-2-oxa-5,8,11-triazatridecan-13-oato(5-)] gadolinate(2-) dihydrogen compound with 1-deoxy-1-(methylamino)-D-glucitol (1:2) with a molecular weight of 1058.2 and an empirical formula of C22H28GdN3O11 • 2C7H17NO5. The structural formula is as follows:
MULTIHANCE has a pH of 6.5-7.5. Pertinent physicochemical parameters are provided below:
Osmolality 1.970 osmol/kg @ 37°C
Viscosity 5.3 mPas @ 37°C
Density 1.220 g/mL @ 20°C
MULTIHANCE has an osmolality 6.9 times that of plasma (285 mOsmol/kg water) and is hypertonic under conditions of use.
CLINICAL PHARMACOLOGY
Gadobenate dimeglumine is a paramagnetic agent and, as such, develops a magnetic moment when placed in a magnetic field. The large magnetic moment produced by the paramagnetic agent results in a large local magnetic field, which can enhance the relaxation rates of water protons in its vicinity leading to an increase of signal intensity (brightness) of tissue.
In magnetic resonance imaging (MRI), visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occur with 1) differences in proton density; 2) differences of the spin-lattice or longitudinal relaxation times (Tl); and 3) differences in the spin-spin or transverse relaxation time (T2). When placed in a magnetic field, gadobenate dimeglumine decreases the T1 and T2 relaxation time in target tissues. At recommended doses, the effect is observed with greatest sensitivity in the Tl-weighted sequences.
Pharmacokinetics
Three single-dose intravenous studies were conducted in 32 healthy male subjects to assess the pharmacokinetics of gadobenate dimeglumine. The doses administered in these studies ranged from 0.005 to 0.4 mmol/kg. Upon injection, the meglumine salt is completely dissociated from the gadobenate dimeglumine complex. Thus, the pharmacokinetics is based on the assay of gadobenate ion, the MRI contrast effective ion in gadobenate dimeglumine. Data for plasma concentration and area under the curve demonstrated linear dependence on the administered dose. The pharmacokinetics of gadobenate ion following intravenous administration can be best described using a two-compartment model.
Distribution: Gadobenate ion has a rapid distribution half-life (reported as mean ± SD) of 0.084 ± 0.012 to 0.605 ± 0.072 hours. Volume of distribution of the central compartment ranged from 0.074 ± 0.017 to 0.158 ± 0.038 L/kg, and estimates of volume of distribution by area ranged from 0.170 ± 0.016 to 0.282 ± 0.079 L/kg. These latter estimates are approximately equivalent to the average volume of extracellular body water in man. In vitro studies showed no appreciable binding of gadobenate ion to human serum proteins.
Metabolism: There was no detectable biotransformation of gadobenate ion. Dissociation of gadobenate ion in vivo has been shown to be minimal, with less than 1% of the free chelating agent being recovered alone in feces.
Elimination: Gadobenate ion is eliminated predominately via the kidneys, with 78% to 96% of an administered dose recovered in the urine. Total plasma clearance and renal clearance estimates of gadobenate ion were similar, ranging from 0.093 ± 0.010 to 0.133 ± 0.270 L/hr/kg and 0.082 ± 0.007 to 0.104 ± 0.039 L/hr/kg, respectively. The clearance is similar to that of substances that are subject to glomerular filtration. The mean elimination half-life ranged from 1.17 ± 0.26 to 2.02 ± 0.60 hours. A small percentage of the administered dose (0.6% to 4%) is eliminated via the biliary route and recovered in feces.
Pharmacokinetics in Special Populations
Renal Impairment: A single intravenous dose of 0.2 mmol/kg of MULTIHANCE was administered to 20 subjects with impaired renal function (6 men and 3 women with moderate renal impairment [urine creatinine clearance >30 to <60 mL/min] and 5 men and 6 women with severe renal impairment [urine creatinine clearance >10 to <30 mL/min]). Mean estimates of the elimination half-life were 6.1 ± 3.0 and 9.5 ± 3.1 hours for the moderate and severe renal impairment groups, respectively as compared with 1.0 to 2.0 hours in healthy volunteers. However, the overall extent of elimination of gadobenate was not influenced by impaired renal function. Also, no differences were noted in renally impaired patients in the rate and type of adverse events reported compared with healthy volunteers, and no deterioration in renal function was observed in this population following the administration of MULTIHANCE. Therefore, dosage adjustment is not recommended(See PRECAUTIONS).
Hemodialysis: A single intravenous dose of 0.2 mmol/kg of MULTIHANCE was administered to 11 subjects (5 males and 6 females) with end-stage renal disease requiring hemodialysis to determine the pharmacokinetics and dialyzability of gadobenate. Approximately 72% of the dose was recovered by hemodialysis over a 4-hour period. The mean elimination half-life on dialysis was 1.21 ± 0.29 hours as compared with 42.4 ± 24.4 hours when off dialysis.
Hepatic Impairment: A single intravenous dose of 0.1 mmol/kg of MULTIHANCE was administered to 11 subjects (8 males and 3 females) with impaired liver function (Class B or C modified Child-Pugh Classification). Hepatic impairment had little effect on the pharmacokinetics of MULTIHANCE with the parameters being similar to those calculated for healthy subjects. (See PRECAUTIONS)
Gender: A multiple regression analysis performed using pooled data from several pharmacokinetic studies found no significant effect of sex upon the pharmacokinetics of gadobenate.
Age: Clearance appeared to decrease slightly with increasing age. Since variations due to age appeared marginal, dosage adjustment for geriatric population is not recommended.
Race: Pharmacokinetic differences due to race have not been systematically studied.
Drug-Drug Interactions: Pharmacokinetic drug interaction studies have not been performed.
Pharmacodynamics
Unlike other paramagnetic contrast agents, MULTIHANCE demonstrates weak and transient interactions with serum proteins that causes slowing in the molecular tumbling dynamics, resulting in strong increases in relaxivity in solutions containing serum proteins. (See Table 1). The improved relaxation effect could potentially contribute to improved visualization.
| Human plasma | |||
| r1 and r2 relaxivities indicate the efficiency in shortening T1 and T2 relaxation times, respectively. | |||
| 1 In heparinized human plasma, at 39°C. | |||
| 2 In citrated human plasma, at 37°C. | |||
| --- Not available | |||
| r1 | r2 | ||
| Gadobenate Gadopentetate Gadodiamide Gadoteridol | 9.71
4.91 5.42 5.42 | 12.51
6.31 --- --- |
|
MULTIHANCE (gadobenate dimeglumine) does not cross the intact blood-brain barrier and, therefore, does not enhance normal brain or lesions that have a normal blood-brain barrier, e.g., cysts, mature post-operative scars, etc. However, disruption of the blood-brain barrier or abnormal vascularity allows enhancement of gadobenate dimeglumine in lesions such as neoplasms, abscesses, and infarcts. Uptake into hepatocytes has been demonstrated for gadobenate. The pharmacokinetics of MULTIHANCE in various lesions is not known.
Effects on Electrocardiography
ECG parameters were investigated in a double-blind, placebo-controlled, 24-hour post dose continuous monitoring, crossover study conducted in 47 subjects (24 healthy volunteers and 23 patients with coronary artery disease [CAD] designed to evaluate the effect of 0.2 mmol/kg MULTIHANCE on ECG intervals, including QTc. Results of the analyses indicate that average changes in QTc values compared with placebo were minimal (< 5 msec). For most individual subjects changes in QTc values were less than 20 msec and evenly distributed between increases and decreases of the same magnitude. QTc prolongation between 30 and 60 msec were noted in 20 subjects (9 healthy volunteers and 11 CAD patients) who received MULTIHANCE vs. 11 subjects (6 volunteers and 5 CAD patients), who received placebo. Prolongations ≥ 61 msec were noted in 6 subjects (2 normal volunteers and 4 CAD patients) who received MULTIHANCE and in 3 subjects (0 volunteers and 3 CAD patients) who received placebo. None of these subjects had associated malignant arrhythmias.
CLINICAL TRIALS
A total of 560 patients were evaluated in 2 controlled clinical trials of the central nervous system (Study A and Study B) with MULTIHANCE. Of these 560 patients, 426 received MULTIHANCE. Of the 426 MULTIHANCE patients, there were 217 men and 209 women with a mean age of 52 years (range 18 to 88 years). The racial and ethnic representations were 88% Caucasian, 6% Black, 4% Hispanic, 1% Asian and 1% other racial or ethnic groups. These trials were designed to evaluate the results of MULTIHANCE contrast MRIs in comparison to non-contrast MRIs alone. In Study A, 410 eligible patients were highly suspected of having a lesion(s) of the CNS based on nuclear medicine imaging, computed tomography (CT), contrast-enhanced CT, MRI, contrast-enhanced MRI, or angiography. After enrollment, patients were randomized to receive two MRI evaluations with 0.05 mmol/kg or 0.1 mmol/kg of MULTIHANCE or with 0.1 mmol/kg of an approved gadolinium contrast agent. Of these 410 patients, 140 received 0.05 mmol/kg of MULTIHANCE, 136 received 0.1 mmol/kg of MULTIHANCE and 134 received an approved gadolinium contrast agent. In Study B, 150 eligible patients had known metastatic disease to the CNS. After enrollment, patients were randomized to receive two MRI evaluations with 0.05 mmol/kg or 0.1 mmol/kg of MULTIHANCE. Of these 150 patients, 74 received 0.05 mmol/kg of MULTIHANCE as the first dose and 76 received 0.1 mmol/kg of MULTIHANCE as the first dose. MRI scans were performed pre-contrast and within 5 minutes after each injection. The studies were designed to evaluate the effect of the first, single dose of MULTIHANCE MRI compared to the non-contrast MRI on a lesion level. Pre-contrast, post-contrast, and pre-plus-post contrast images (paired images) were independently evaluated by three blinded readers. The images were evaluated for the following endpoints using a scale from 0 to 4: the degree of lesion border delineation, the degree of visualization of lesion internal morphology, and the degree of lesion contrast enhancement. Lesion counting was also performed for the pre-contrast and paired image sets. The pre-contrast versus post-contrast comparison on a lesion level was prospective whereas, the pre-contrast versus paired comparison was ad hoc.
In the prespecified pre-contrast versus post-contrast comparisons, the mean score differences between the pre-contrast and the post-contrast were significant for subjects in Study B (all subjects with known metastatic lesions) and for a subset of subjects with known tumors in Study A. However, the mean score differences between the pre-contrast and post-contrast comparisons were not significant for the subset of non-tumor patients in Study A. These negative results may be attributed to a lack of lesion enhancement for these patients’ underlying non-tumor CNS disease.
As shown in Table 2, the first row of each endpoint group represents the difference in the mean score of the paired MRI reads from the mean score of the pre-contrast MRI reads alone on a lesion level analysis. Also, the table shows the number of lesions whose paired MRI images were read as better, worse, or the same as the pre-contrast MRI images. In Table 2 for these endpoints, when read in combination with the non-contrast images, 0.1 mmol/kg MULTIHANCE provided a statistically significant improvement over baseline. Also, more lesions were seen in the paired images than in the pre-contrast images alone. With the 0.1 mmol/kg dose, the images demonstrated consistently better visualization for all readers for all visualization endpoints. However, the 0.05 mmol/kg dose provided inconsistent visualization between readers.
An additional analysis of the difference in the mean score of the post contrast MRI reads from the mean score of the pre-contrast MRI reads alone for the three endpoints on a patient level (the mean score across all lesions within a patient) is shown in Table 3. For these endpoints, 0.1 mmol/kg MULTIHANCE provided a statistically significant improvement over baseline.
| Study A | Study B | |||||
| (a) Difference of means = (paired mean) - (pre mean) | ||||||
| (b) Worse = paired score is less than the pre score Same = paired score is the same as the pre score Better = paired score is greater than the pre score |
||||||
| (c) Paired = side-by-side pre and post MULTIHANCE | ||||||
| * Statistically significant for the mean (paired t test) | ||||||
| Reader 1 | Reader 2 | Reader 3 | Reader 1 | Reader 2 | Reader 3 | |
| Endpoints | N = 395 | N = 384 | N = 299 | N = 245 | N = 275 | N = 254 |
| Border Delineation: Difference of Means (a) | 0.8* | 0.6* | 0.8* | 1.8* | 1.5* | 1.9* |
| Worse (b) Same Better | 44 (11%) 146 (37%) 205 (52%) | 61 (16%) 168 (44%) 155 (40%) | 57 (19%) 89 (30%) 153 (51%) | 13 (5%) 11 (5%) 221 (90%) | 24 (9%) 19 (7%) 232 (84%) | 15 (6%) 18 (7%) 221 (87%) |
| Internal Morphology: Difference of Means | 0.8* | 0.6* | 0.7* | 1.7* | 1.4* | 2.1* |
| Worse Same Better | 37 (10%) 147 (37%) 211 (53%) | 63 (17%) 151 (39%) 170 (44%) | 62 (21%) 84 (28%) 153 (51%) | 13 (5%) 16 (7%) 216 (88%) | 26 (10%) 22 (8%) 227 (82%) | 14 (5%) 22 (9%) 218 (86%) |
| Contrast Enhancement: Difference of Means | 0.7* | 0.5* | 0.8* | 1.9* | 1.3* | 1.9* |
| Worse Same Better | 75 (19%) 148 (37%) 172 (44%) | 74 (19%) 152 (40%) 158 (41%) | 50 (17%) 109 (36%) 140 (47%) | 13 (5%) 11 (5%) 221 (90%) | 32 (12%) 21 (7%) 222 (81%) | 17 (7%) 14 (5%) 223 (88%) |
| Study A | Study B | |||||
| (a) Difference of means = (post MULTIHANCE mean) - (pre mean) | ||||||
| † Endpoints are on a patient level, with each endpoint being the mean score across all lesions within a patient | ||||||
| * Statistically significant for the mean (paired t test) | ||||||
| Reader 1 | Reader 2 | Reader 3 | Reader 1 | Reader 2 | Reader 3 | |
| Endpoints† | N = 78 | N = 73 | N = 70 | N = 65 | N = 71 | N = 69 |
| Border Delineation: Difference of Means (a) | 0.5* | 0.6* | 0.5* | 1.4* | 1.1* | 1.2* |
| Internal Morphology: Difference of Means | 0.5* | 0.7* | 0.5* | 1.2* | 0.8* | 1.0* |
| Contrast Enhancement: Difference of Means | 0.3* | 0.5* | 0.4* | 1.5* | 0.9* | 1.2* |
INDICATIONS AND USAGE
MULTIHANCE is indicated for intravenous use in magnetic resonance imaging (MRI) of the CNS in adults to visualize lesions with abnormal blood brain barrier or abnormal vascularity of the brain, spine, and associated tissues.
CONTRAINDICATIONS
MULTIHANCE is contraindicated in patients with known allergic or hypersensitivity reactions to gadolinium or any other ingredients, including benzyl alcohol.
WARNINGS
Nephrogenic Systemic Fibrosis (NSF)
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate <30 mL/min/1.73m2) and in patients with acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period. In these patients, avoid use of gadolinium-based contrast agents unless the diagnostic information is essential and not available with non-contrast enhanced MRI. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a gadolinium-based contrast agent in order to enhance the contrast agent’s elimination. The usefulness of hemodialysis in the prevention of NSF is unknown.
Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a gadolinium-based contrast agent and the degree of renal function impairment at the time of exposure.
Post-marketing reports have identified the development of NSF following single and multiple administrations of gadolinium-based contrast agents. These reports have not always identified a specific agent. Where a specific agent was identified, the most commonly reported agent was gadodiamide (OmniscanTM), followed by gadopentetate dimeglumine (Magnevist®) and gadoversetamide (OptiMARK®). NSF has also developed following sequential administrations of gadodiamide with gadobenate dimeglumine (MultiHance®) or gadoteridol (ProHance®). The number of post-marketing reports is subject to change over time and may not reflect the true proportion of cases associated with any specific gadolinium-based contrast agent.
The extent of risk for NSF following exposure to any specific gadolinium-based contrast agent is unknown and may vary among the agents. Published reports are limited and predominantly estimate NSF risks with gadodiamide. In one retrospective study of 370 patients with severe renal insufficiency who received gadodiamide, the estimated risk for development of NSF was 4% (J Am Soc Nephrol 2006;17:2359). The risk, if any, for the development of NSF among patients with mild to moderate renal insufficiency or normal renal function is unknown.
Screen all patients for renal dysfunction by obtaining a history and/or laboratory tests. When administering a gadolinium-based contrast agent, do not exceed the recommended dose and allow a sufficient period of time for elimination of the agent prior to any readministration. (See CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Deoxygenated sickle erythrocytes have been shown in in vitro studies to align perpendicular to a magnetic field which may result in vaso-occlusive complications in vivo. The enhancement of magnetic moment by MULTIHANCE may possibly potentiate sickle erythrocyte alignment. MULTIHANCE has not been studied in patients with sickle cell anemia and other hemoglobinopathies. Patients with other hemolytic anemias have not been adequately evaluated following administration of MULTIHANCE to exclude the possibility of increased hemolysis.
Patients with a history of allergy, drug reactions, or other hypersensitivity-like disorders should be closely observed during the procedure and for several hours after drug administration. (See PRECAUTIONS - General)
PRECAUTIONS
General
Diagnostic procedures that involve the use of contrast agents should be carried out under direction of a physician with the prerequisite training and a thorough knowledge of the procedure to be performed.
Although more lesions are generally visualized on contrast-enhanced images than on unenhanced images, lesions seen on unenhanced images may not all be seen on contrast-enhanced images. CAUTION SHOULD BE EXERCISED WHEN A CONTRAST-ENHANCED INTERPRETATION IS MADE IN THE ABSENCE OF A COMPANION UNENHANCED MRI.
Appropriate facilities should be available for coping with any complications of the procedures, as well as for emergency treatment of severe reactions to the contrast itself. The possibility of a reaction, including serious, life-threatening, or fatal, anaphylactic or cardiovascular reactions, or other idiosyncratic reactions, should always be considered, especially in those patients with a history of a known clinical hypersensitivity or a history of asthma or other allergic respiratory disorders.
Injection site reactions
In rabbits, perivenous injection of MULTIHANCE provoked more severe local reactions than intravenous injection in rabbits. In these animal experiments, local reactions including eschar and necrosis were noted even on Day 8 post perivenous injection of MULTIHANCE. Therefore, caution should be exercised to avoid local extravasation during intravenous administration of MULTIHANCE. If extravasation occurs, subjects should be monitored and treated as necessary if local reactions develop.
Electrocardiographic Changes
The effects of QTc by dose, other drugs, and medical conditions were not systematically studied. Several atrial and ventricular arrhythmias and atrio-ventricular conduction defects were observed in subjects who received MULTIHANCE. Caution should be exercised in patients who may be using medications or who may have underlying metabolic, cardiac, or other abnormalities that may predispose to cardiac arrhythmias. (see ADVERSE REACTIONS BELOW).
Drug Interactions
MULTIHANCE and other drugs may compete for the cannalicular multispecific organic anion transporter (cMOAT also referred to as MRP2 or ABCC2) sites. Therefore appropriate caution should be exercised in those patients who receive drugs such as cisplatin, antracyclines (such as doxorubicin, daunorubicin), vinca alkaloids (such as vincristine), methotrexate, etoposide, tamoxifen, taxol (pacilitaxel), or others. Caution should also be exercised in those subjects in whom the cMOAT sites may be affected due to underlying metabolic disorders such as Dubin Johnson syndrome, etc. (see also Laboratory Test Interactions below).
Laboratory Test Interactions
Transient increases in serum ferritin were observed in some patients that were attributed to the underlying disease. In patients with renal disease, transient increases in urine zinc were detected and these changes were shown not to be clinically significant. Transient asymptomatic elevations in bilirubin over baseline were observed in patients with underlying hepatic metabolic disorders such as von Willebrands’ disease and Wilsons’ disease.
Information for Patients
Patients scheduled to receive MULTIHANCE should be instructed to inform their physician if the patient:
- is pregnant or breast feeding.
- has anemia or diseases that affect the red blood cells.
- has a history of renal disease, heart disease, seizure, hemoglobinopathies, or asthma or allergic respiratory diseases.
- is taking any medications.
- has any allergies to any of the ingredients of MULTIHANCE.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of MULTIHANCE.
The results for MULTIHANCE were negative in the following genetic toxicity studies: 1) in vitro bacteria reverse mutation assays, 2) an in vitro gene mutation assay in mammalian cells, 3) anin vitro chromosomal aberration assay, 4) an in vitro unscheduled DNA synthesis assay, and 5) an in vivo micronucleus assay in rats.
MULTIHANCE had no effect on fertility and reproductive performance at IV doses of up to 2 mmol/kg/day (3 times the human dose on body surface basis) for 13 weeks in male rats and for 32 days in female rats. However, vacuolation in testes and abnormal spermatogenic cells were observed when MULTIHANCE was intravenously administered to male rats at 3 mmol/kg/day (5 times the human dose on body surface basis) for 28 days. The effects were not reversible following 28-day recovery period. The effects were not reported in dog and monkey studies (at doses up to about 11 and 10 times the human dose on body surface basis for dogs (28 days dosing) and monkeys (14 days dosing), respectively).
Pregnancy
Pregnancy Category C
MULTIHANCE has been shown to be teratogenic in rabbits when given intravenously administered at 2 mmol/kg/day (6 times the human dose based on body surface area) during organogenesis (day 6 to 18) inducing microphthalmia / small eye and / or focal retinal fold in 3 fetuses from 3 separate litters. In addition, MULTIHANCE intravenously administered at 3 mmol/kg/day (10 times the human dose based on body surface area) has been shown to increase intrauterine deaths in rabbits. There was no evidence that MULTIHANCE induced teratogenic effects in rats at doses up to 2 mmol/kg/day (3 times the human dose based on body surface area), however, rat dams exhibited no systemic toxicity at this dose. There were no adverse effects on the birth, survival, growth, development and fertility of the F1 were no adverse effects on the birth, survival, growth, development and fertility of the F1 were no adverse effects on the birth, survival, growth, development and fertility of the F1 generation at doses up to 2 mmol/kg in a rat peri- and post-natal (Segment III) study. There are no adequate and well-controlled studies in pregnant women. MULTIHANCE should be used during pregnancy only if potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known to what extent gadobenate dimeglumine is excreted in human milk. It is known from rat experiments that less than 0.5% of the administered dose is transferred via milk from mother to neonates. Breast-feeding should be discontinued prior to the administration of MULTIHANCE and should not be restarted until at least 24 hours after the administration of MULTIHANCE.
Geriatric Use
Of the total number of 2982 adult subjects in clinical studies of MULTIHANCE, 27% were 65 and over. No overall differences in safety or effectiveness were observed between these elderly subjects and the younger subjects.
The drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to MULTIHANCE may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function it may be useful to monitor renal function.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
In clinical trials, a total of 2982 adult subjects (119 healthy volunteers and 2863 patients) received MULTIHANCE at doses ranging from 0.005 to 0.4 mmol/kg. There were 1724 (58%) men and 1258 (42%) women with a mean age of 55.1 years (range 18 to 92 years). A total of 2644 (89%) subjects were Caucasian, 84 (3%) Black, 162 (5%) Asian, 29 (1%) Hispanic, 18 (1%) in other racial groups, and for 45 (2%) subjects, race was not reported. Among the 2863 patients, 65 subjects were adult patients who participated in special population pharmacokinetics or cardiac electrophysiology studies (n = 20, renal impairment patients; n = 11, renal dialysis patients; n = 11, hepatic impairment patients; n = 23, ECG cardiovascular patients).
Of the 2982 adult subjects who received MULTIHANCE, 531 (17.8%) reported at least one adverse event. In comparison, 35 (27.6%) of the 127 subjects (38 healthy volunteers and 89 patients) who received placebo in clinical trials reported at least one adverse event.
The most commonly reported adverse events in adult subjects who received MULTIHANCE were headache (2.2%) and nausea (1.8%). Most adverse events were mild to moderate in intensity. Two subjects (0.1 %) died and in 13 additional subjects (0.4%), 15 serious adverse events were reported. The two deaths were attributable to the patients’ underlying medical conditions. In four of the 13 subjects who experienced serious adverse events, a causal relationship to MULTIHANCE could not be excluded. One subject with a history of seizures experienced convulsions 17 minutes after the administration of MULTIHANCE. Another subject with a history of recent MI and possibly CHF experienced acute pulmonary edema within 10 minutes after the administration of 30 mL of MULTIHANCE. In the third subject who developed acute necrotizing pancreatitis, sufficient information was not available to exclude a causal relationship to MULTIHANCE. Anaphylactoid reaction was suspected in the fourth subject who experienced laryngismus in conjunction with dyspnea. (See WARNINGS and PRECAUTIONS, General). The incidence of adverse events for a subgroup of adult patients with known or suspected lesions of the CNS who participated in Study A (See CLINICAL TRIALS) was comparable among the 276 patients who received MULTIHANCE (28.6%), and the 134 patients who received an approved gadolinium contrast agent (32.1%). The most commonly reported adverse events in patients who received MULTIHANCE for CNS imaging were headache (5.8%), dizziness (3.6%), and taste perversion (3.3%). The other adverse events that were reported in patients who received MULTIHANCE are similar in nature to those reported in the adult population as a whole. Adverse events that occurred in at least 0.5% of 2982 adult subjects who received MULTIHANCE are listed below in related categories, in decreasing order of occurrence within each system, and regardless of causality. The incidence for placebo-treated subjects and the CNS subpopulation are also shown for purposes of comparison.
| All Adult Subjects | CNS Studies |
||
| Placebo | MULTIHANCE | MULTIHANCE | |
| Number of subjects dosed | 127 | 2982 | 659 |
| Number of subjects with any adverse event | 35 (27.6%) | 531 (17.8%) | 148 (22.5%) |
| Body as a Whole Headache Injection site reaction Pain |
6 (4.7%) 4 (3.1%) 0 |
67 (2.2%) 44 (1.5%) 19 (0.6%) |
25 (3.8%) 8 (1.2%) 2 (0.3%) |
| Cardiovascular System Hypertension Tachycardia |
4 (3.1%) 1 (0.8%) |
22 (0.7%) 14 (0.5%) |
2 (0.3%) 2 (0.3%) |
| Digestive System Nausea Vomiting Diarrhea |
2 (1.6%) 1 (0.8%) 3 (2.4%) |
55 (1.8%) 16 (0.5%) 14 (0.5%) |
12 (1.8%) 4 (0.6%) 1 (0.2%) |
| Hemic and Lymphatic System Anemia |
0 |
16 (0.5%) |
3 (0.5%) |
| Nervous System Vasodilatation Paresthesia Dizziness |
1 (0.8%) 2 (1.6%) 2 (1.6%) |
31 (1. 0%) 24 (0.8%) 22 (0.7%) |
8 (1.2%) 3 (0.5%) 10 (1.5%) |
| Skin and Appendages Rash |
2 (1.6%) |
21 (0.7%) |
4 (0.6%) |
| Special Senses Taste perversion |
3 (2.4%) |
25 (0.8%) |
9 (1.4%) |
Adverse reactions that occurred in less than 0.5% of the 2982 adult subjects who received MULTIHANCE included:
Body as a Whole: Abdominal pain, anaphylactic reaction, asthenia, back pain, chest pain, chills, facial edema, fever, infection, infiltration of contrast, injection site inflammation, injection site pain, malaise.
Cardiovascular System: Acute pulmonary edema, arrhythmia, atrial fibrillation, bradycardia, ECG abnormality (includes bundle branch block, complete AV block, first-degree AV block, inverted T wave, prolonged PR interval, prolonged QT interval, shortened QT interval), hypotension, myocardial ischemia, palpitations, supraventricular extrasystoles, syncope, ventricular arrhythmia, ventricular extrasystoles (See PRECAUTIONS).
Digestive System: Constipation, dyspepsia, fecal incontinence, acute necrotizing pancreatitis, increased pruritus in patients with cirrhosis.
Hemic and Lymphatic System: Basophilia, hemolysis, leukocytosis, leukopenia.
Metabolic and Nutritional System: Abnormal laboratory test (includes changes in CPK, creatinine, ferritin, transferrin, total iron binding capacity), bilirubinemia, hyperglycemia, hyperkalemia, hyperlipemia, hypocalcemia, hypoglycemia, hyponatremia, hypoproteinemia, increased alkaline phosphatase, increased GGT, increased LDH, increased serum iron, increased SGOT, increased SGPT, peripheral edema, thirst.
Musculoskeletal System: Myalgia, myositis.
Nervous System: Cold feeling, convulsion, dry mouth, hemiplegia, hypertonia, hypesthesia, increased salivation, paralysis, stupor, tremor, aphasia.
Respiratory System: Dyspnea, hyperventilation, increased cough, laryngismus, lung edema, rhinitis, pulmonary embolus.
Skin and Appendages: Pruritus, sweating, urticaria.
Special Senses: Abnormal vision, ear pain, eye disorder, parosmia, tinnitus.
Urogenital System: Albuminuria, glycosuria, hematuria, urinary frequency, urinary incontinence, urinary tract infection, urinary urgency.
Non US Post Marketing Experience: There were reports of anaphylactoid reactions (characterized by cardiovascular, respiratory, and/or cutaneous symptoms) anaphylactic shock, and loss of consciousness. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
OVERDOSAGE
Clinical consequences of overdosage with MULTIHANCE have not been reported. Treatment of an overdosage should be directed toward support of vital functions and prompt institution of symptomatic therapy. In a Phase I clinical study, doses up to 0.4 mmol/kg were administered to patients. MULTIHANCE has been shown to be dialyzable. (See CLINICAL PHARMACOLOGY - Pharmacokinetics.)
DOSAGE AND ADMINISTRATION
The recommended dose of MULTIHANCE is 0.1 mmol/kg (0.2 mL/kg) administered as a rapid bolus intravenous injection.
To ensure complete injection of the contrast medium, the injection should be followed by a saline flush of at least 5 mL. It is important to ensure that the i.v. needle or cannula is correctly inserted into a vein.
Parenteral products should be inspected visually for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored or particulate matter is present. Concurrent medications or parenteral nutrition should not be physically mixed with contrast agents and should not be administered in the same intravenous line because of the potential for chemical incompatibility.
When MULTIHANCE injection is to be injected using plastic disposable syringes, the contrast should be drawn into the syringe and used immediately.
MULTIHANCE injection should be drawn into the syringe and administered using sterile technique. If non-disposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents. Any residual product must be discarded in accordance with regulations dealing with the disposal of such materials.
HOW SUPPLIED
MULTIHANCE (gadobenate dimeglumine) is a clear, colorless solution containing 529 mg gadobenate dimeglumine per mL. MULTIHANCE is supplied in glass vials; each single dose vial is rubber stoppered with an aluminum seal and the contents are sterile. MULTIHANCE is supplied in boxes of:
Five 5 mL single dose 10 mL vials (NDC 0270-5164-12)
Five 10 mL single dose 20 mL vials (NDC 0270-5164-13)
Five 15 mL single dose 20 mL vials (NDC 0270-5164-14)
Five 20 mL single dose 20 mL vials (NDC 0270-5164-15)
STORAGE
Store at 25°C (77°F), excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Do not freeze.
US Patent No. 4,916,246
Manufactured for
Bracco Diagnostics Inc. - Princeton, NJ 08543
By ALTANA Pharma AG - 78224 Singen (Germany)
Revised May 2007
F1/3.5524.62
| MultiHance (gadobenate dimeglumine) | |||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Revised: 02/2008Bracco Diagnostics Inc.