BREVOXYL
-
benzoyl peroxide cream
Physicians Total Care, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION
BREVOXYL-4® Creamy Wash and BREVOXYL®-8 Creamy Wash are topical preparations containing benzoyl peroxide as the active ingredient. BREVOXYL®-4 Creamy Wash and BREVOXYL®-8 Creamy Wash contain: 4% and 8% Benzoyl Peroxide, respectively, in a lathering cream vehicle containing Cetearyl Alcohol, Cocamidopropyl Betaine, Dimethyl Isosorbide, Glycerin, Glycolic Acid, Hydrogenated Castor Oil, Imidurea, Lactic Acid, Methylparaben, Mineral Oil, PEG-14M, Potassium Lauryl Sulfate, Potassium Phosphate, Purified Water, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium PCA, Titanium Dioxide, Zea Mays (Corn) Starch.
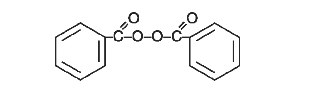
The structural formula of benzoyl peroxide is:
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris in not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide is believed to be responsible for its usefulness in acne.
Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
INDICATIONS AND USAGE
BREVOXYL®-4 CreamyWash and BREVOXYL®-8 Creamy Wash are indicated for use in the topical treatment of mild to moderate acne vulgaris. BREVOXYL®-4 Creamy Wash and BREVOXYL®-8 Creamy Wash may be used as an adjunct in acne treatment regimens including antibiotics, retinoic acid products, and sulfur/salicylic acid containing preparations.
CONTRAINDICATIONS
BREVOXYL®-4 Creamy Wash and BREVOXYL®-8 Creamy Wash should not be used in patients who have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the product.
PRECAUTIONS
General
For external use only. Avoid contact with eyes and mucous membranes. AVOID CONTACT WITH HAIR, FABRICS OR CARPETING AS BENZOYL PEROXIDE WILL CAUSE BLEACHING. Intensive sun bathing or UV radiation should be avoided.
Carcinogenesis, Mutagenesis, Impairment of Fertiliy
Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
Pregnancy
Category C
Animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed.
ADVERSE REACTIONS
The major adverse reaction reported to date with BREVOXYL® -4 Creamy Wash and BREVOXYL® - 8 Creamy Wash therapy is irritation of the skin including erythema, burning, peeling, dryness, and pruritus. This is reversible when treatment is reduced in frequency or discontinued. Dermatitis and allergic contact dermatitis including face edema may occur.
OVERDOSE
BREVOXYL® - 4 Creamy Wash and BREVOXYL® - 8 Creamy Wash are for topical use only. If the medication is applied excessively, no more rapid or better results will be obtained, and undesirable effects might develop, such as severe irritation. In this event, discontinue use of the product and wait until the skin has recovered its normal aspect. Cold compresses can provide relief from irritation.
DOSAGE AND ADMINISTRATION
Shake well before using. Wash the affected areas once a day during the first week, and twice a day thereafter as tolerated. A mild burning sensation may occur on first application. Wet skin areas to be treated; apply BREVOXYL® - 4 Creamy Wash or BREVOXYL® - 8 Creamy Wash, work to a full later, rinse thoroughly and pat dry. Frequency of use should be adjusted to obtain the desired clinical response. Clinically visible improvement will normally occur by the third week of therapy. Maximum lesion reduction may be expected after approximately eight to twelve weeks of drug use. Continuing use of the drug is normally required to maintain a satisfactory clinical response.
HOW SUPPLIED
BREVOXYL® - 4 Creamy Wash is supplied in
- 170.1 g (6.0 oz) tubes NDC 54868-2248-0
Store at controlled room temperature, 15º – 30ºC (59º – 86ºF).
Questions? call 1-888-500-DERM (3376). Serious side effects associated with use of this product may be reported to this number.
STIEFEL®
Stiefel Laboratories, Inc.
255 Alhambra Circle
Coral Gables, FL 33134-7412 USA
BREVOXYL, PANOXYL, STIEFEL and STIEFEL & Design are registered trademarks of Stiefel Laboratories, Inc.
© 2009 Stiefel Laboratories, Inc.
U.S. Patent No. 6,433,024
302995
July 2009
Additional barcode labeling by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
Principal Display Panel
NDC 54868-2248-0
Brevoxyl®-4
Creamy Wash
(benzoyl peroxide 4%)
FOR TOPICAL USE
Rx only
Net Wt
170.1 g (6.0 oz)
| BREVOXYL
benzoyl peroxide cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 03/03/2005 | 12/31/2006 | |
| Labeler - Physicians Total Care, Inc. (194123980) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Physicians Total Care, Inc. | 194123980 | relabel | |
Revised: 03/2012 Physicians Total Care, Inc.