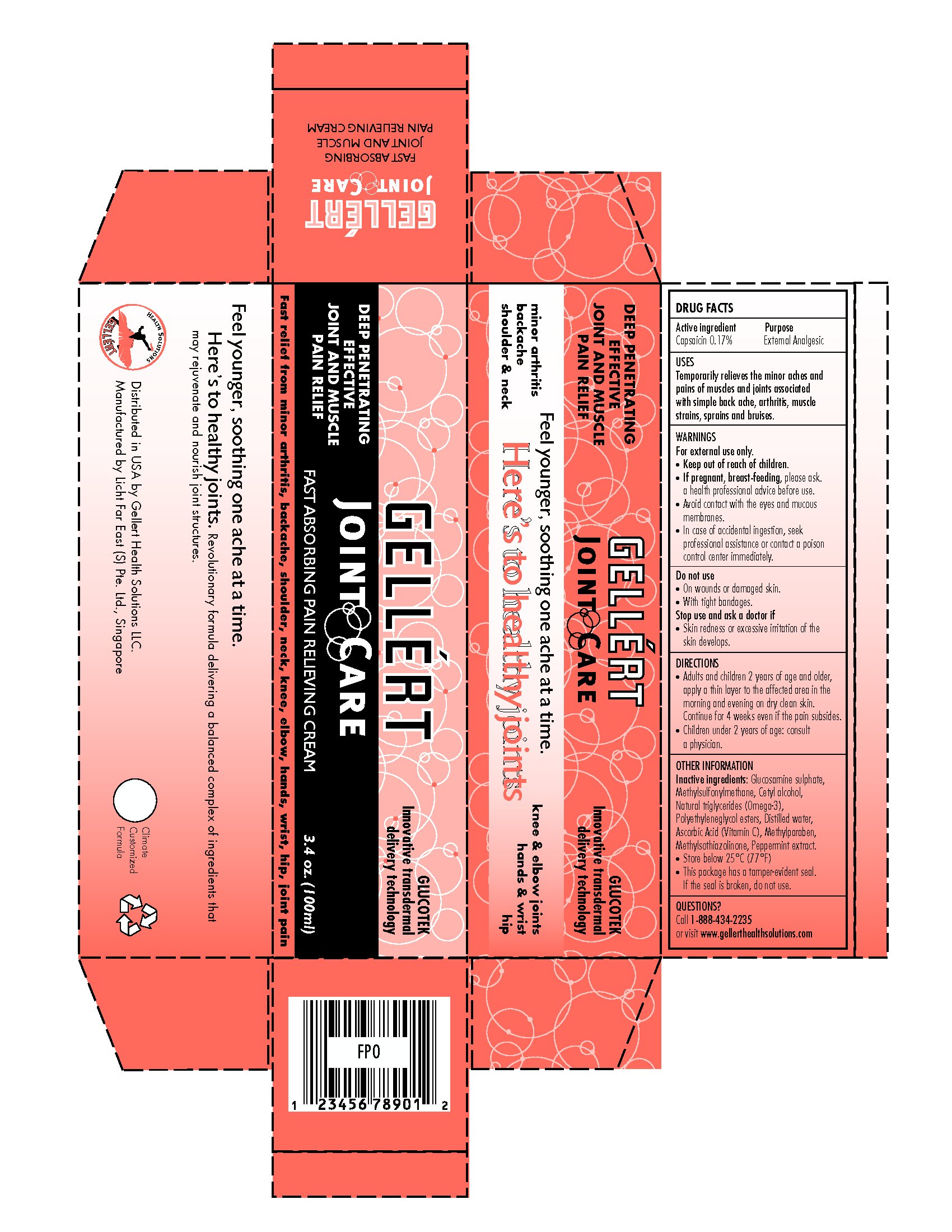
GELLERT JOINT CARE
-
capsaicin cream
Gellert Health Solutions LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Capsaicin 0.17%
Purpose
External Analgesic
For External Use Only
Avoid contact with the eyes and mucous membranes.
In case of accidental ingestion, seek professional assistance or contact a poison control center immediately.
Keep out of reach of children.
Stop use and ask a doctor if
- Skin redness or excessive irritation of the skin develops.
Do Not Use:
- On wounds or damaged skin.
- With tight bandages
Directions
- Adults and children 2 years of age and older, apply a thin layer to the affected area in the morning and evening on dry clean skin. Continue for 4 weeks even if the pain subsides.
- Children under 2 years of age: consult a physician.
Inactive ingredients:
Glucosamine sulphate, Methylsulfonylmethane, Cetyl alcohol, Natural triglycerides (Omega-3), Polyethyleneglycol esters, Distilled water, Ascorbic Acid (Vitamin C), Methylparaben, Methylsothiazolinone, Peppermit extract.
Other Information
- Store below 25oC (77oF).
- This package has a tamper-evident seal. If the seal is broken, do not use.
Questions?
Call: 1-888-434-2235
or visit www.gellerthealthsolutions.com
GELLERT JOINT CARE
capsaicin cream |
|
|
|
|
|
|
|
|
|
|
Revised: 03/2012 Gellert Health Solutions LLC