CLINAC BPO 7
-
benzoyl peroxide gel
Ferndale Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION
Clinac® BPO 7 (benzoyl peroxide) is a topical preparation containing 7% benzoyl peroxide as the active ingredient in a gel vehicle containing purified water, propylene glycol, Acrysorb® (brand of acrylates copolymer), PEG-400, carbomer 940, disodium EDTA, and sodium hydroxide.
The chemical structure for the active ingredient is:
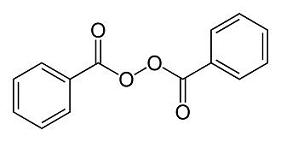
benzoyl peroxide
C14H10O4 (anhydrous) 242.23
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl Peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action combines with the mild keratolytic effect of benzoyl peroxide is believed to be responsible for its usefulness in acne.
Little is known about the percutaneous penetration, metabolism and excretion of benzoyl peroxide. Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine. There is no evidence of systemic toxicity caused by benzoyl peroxide in humans.
INDICATIONS AND USAGE
Clinac® BPO 7 is indicated for the topical treatment of mild to moderate acne vulgaris.
CONTRAINDICATIONS
Clinac® BPO 7 should not be used in patients who have shown hypersensitivity to benzoyl peroxide or any of the other ingredients in this product.
WARNINGS
FOR EXTERNAL USE ONLY. KEEP OUT OF REACH OF CHILDREN. Avoid contact with eyes, eyelids, lips and mucous membranes. If inadvertent contact occurs, rinse area thoroughly with water. Contact with colored material, including fabric and hair, may result in discoloration. When using Clinac® BPO 7, avoid unnecessary sun exposure and use a sunscreen. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately.
PRECAUTIONS
Information for Patients
- Avoid contact with eyes, eyelids, lips and mucous membranes.
- May discolor hair and fabrics.
- Avoid unnecessary sun exposure and use a sunscreen.
- Use of oil-free make-up recommended.
- If severe irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Studies employing a strain of mice that are highly susceptible to developing cancer suggest that benzoyl peroxide act as a tumor promoter. The clinical significance of these finding to humans is not known. Benzoyl peroxide has not been found to be mutagenic (Ames test) and there are no published data indicating that it impairs fertility.
Pregnancy: Category C
Animal reproductive studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed. There are no available data on the effect of benzoyl peroxide on the later growth, development and functional maturation of the unborn child.
ADVERSE REACTIONS
Allergic contact dermatitis and dryness have been reported with topical benzoyl peroxide therapy. If excessive scaling, erythema or edema occurs, the use of Clinac® BPO 7 should be discontinued and appropriate therapy instituted. To hasten resolution of adverse effects, cool compresses may be used. After reaction clears, a reduced dosage schedule may often be resumed if the reaction is judged not to be due to allergenicity.DOSAGE AND ADMINISTRATION
Wash and dry hands, face and affected areas with a gentle cleanser before application. Apply a thin layer of Clinac® BPO 7 to the affected area once or twice daily, or as directed by your physician. Replace cap tightly after use.
HOW SUPPLIED
Clinac® BPO 7 Gel 45 gram tube NDC 0496-0857-45
Store at 25°C (77°F); excusions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature.].
Rx Only.
Ferndale Laboratories, Inc.
Ferndale, MI 48220 U.S.A
Toll free (888) 548-0900
www.ferndalelabs.com
Protected under U.S. Patent.
Clinac® and Acrysorb® are registered trademarks of Dow Pharmaceutical Sciences Corp.
| CLINAC BPO 7
benzoyl peroxide gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 09/12/2000 | 11/30/2012 | |
| Labeler - Ferndale Laboratories, Inc. (005320536) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ferndale Laboratories, Inc. | 005320536 | manufacture | |
Revised: 03/2012 Ferndale Laboratories, Inc.
