DE LA CRUZ DOLOFEBRIL INFANT
-
acetaminophen suspension
DLC Laboratories, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
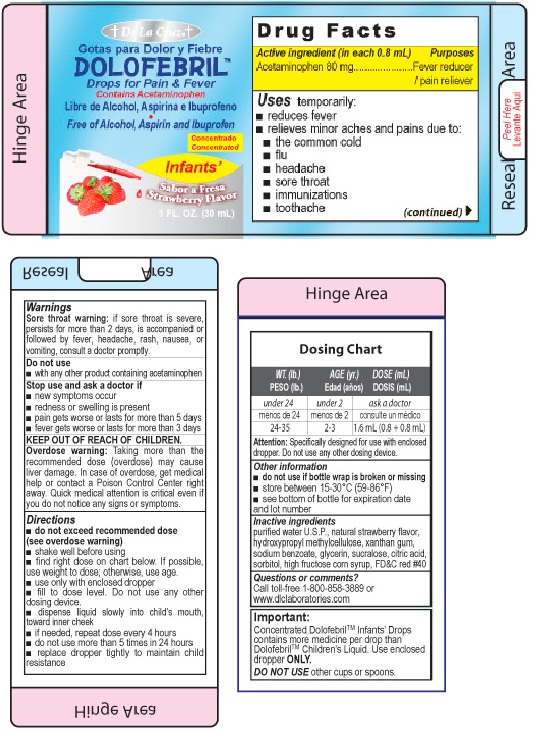
Uses:
temporarily:
- reduces fever
- relieves minor aches and pains due to:
-
- the common cold
- flu
- headache
- sore throat
- immunizations
- toothache
Warnings
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
Directions
- Do not exceed recommended dose (see overdose warning)
- Shake well before using
- find right dose on chart below. If possible, use weight to dose; otherwise use age
- if needed, repeat dose every 4 hours
- do not use more than 5 times in 24 hours
- only use enclosed measuring cup
| Weight (lb)
| Age (Years) | Dose (teaspoons |
| under 24 | under 2 | ask a doctor |
| 24-35 | 2-3 | 1.6mL (0.8 + 0.8mL) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Information
Each teaspoon contains: sodium 2mg
Do not use if bottle wrap is broken or missing
Store between 15-20 degreesC (59-86degreesC)
see bottom panel for lot number and exipiration date.
Inactive Ingredients
purified water U.S.P., natural strawberry flavor, hydroxypropyl methylcellulose, xanthan gum, sodium benzoate, glycerin, sucralose, citric acid, sorbitol, high fructose corn syrup, FDandC Red No. 40
Package Labeling
Dolofebril
For Pain and Fever
Free of Alcohol, Aspirin and Ibuprofen
Infants
Strawberry Flavor
| DE LA CRUZ DOLOFEBRIL INFANT
acetaminophen suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part343 | 02/27/2012 | 02/27/2012 |
| Labeler - DLC Laboratories, Inc (093351930) |
Revised: 02/2012 DLC Laboratories, Inc