flarex (fluorometholone acetate) suspension
[Alcon Laboratories, Inc.]
DESCRIPTION
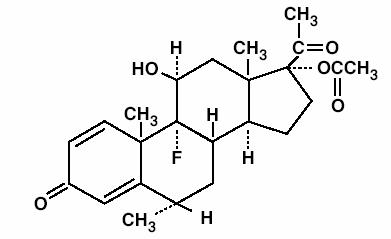
FLAREX® (fluorometholone acetate) is a corticosteroid prepared as a sterile topical ophthalmic suspension. The active ingredient, fluorometholone acetate, is a white to creamy white powder with an empirical formula of C24H31FO5 and a molecular weight of 418.5. Its chemical name is 9-fluoro-11β, 17-dihydroxy-6α -methylpregna-1, 4-diene-3, 20-dione 17-acetate. The chemical structure of Fluorometholone Acetate is presented below:
Each mL contains: Active: fluorometholone acetate 1 mg (0.1%). Preservative: benzalkonium chloride 0.01%. Inactive: sodium chloride, monobasic sodium phosphate, edetate disodium, hydroxyethyl cellulose, tyloxapol, hydrochloric acid and/or sodium hydroxide (to adjust pH), and purified water. DM-00
CLINICAL PHARMACOLOGY
Corticosteroids suppress the inflammatory response to inciting agents of mechanical, chemical or immunological nature. No generally accepted explanation of this steroid property has been advanced. Clinical studies demonstrate that Fluorometholone Acetate is significantly more efficacious than Fluorometholone for the treatment of external ocular inflammation.1 Corticosteroids cause a rise in intraocular pressure in susceptible individuals. In a small study, FLAREX Ophthalmic Suspension demonstrated a significantly longer average time to produce a rise in intraocular pressure than did dexamethasone phosphate; however, the ultimate magnitude of the rise was equivalent for both drugs and in a small percentage of individuals a significant rise in intraocular pressure occurred within three days.2
INDICATIONS AND USAGE
FLAREX Ophthalmic Suspension is indicated for use in the treatment of steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye.
CONTRAINDICATIONS
Contraindicated in acute superficial herpes simplex keratitis, vaccinia, varicella, and most other viral diseases of cornea and conjunctiva; tuberculosis; fungal diseases; acute purulent untreated infections which, like other diseases caused by microorganisms, may be masked or enhanced by the presence of the steroid; and in those persons who have known hypersensitivity to any component of this preparation.
WARNINGS
Not for injection. Use in the treatment of herpes simplex infection requires great caution. Prolonged use may result in glaucoma, damage to the optic nerve, defect in visual acuity and visual field, cataract formation and/or may aid in the establishment of secondary ocular infections from pathogens due to suppression of host response. Acute purulent infections of the eye may be masked or exacerbated by presence of steroid medication. In those diseases causing thinning of the cornea or sclera, perforation has been known to occur with chronic use of topical steroids. It is advisable that the intraocular pressure be checked frequently.
PRECAUTIONS
General
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local steroid application. Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the suspension. The preservative in Flarex®, benzalkonium chloride, may be absorbed by soft contact lenses. Flarex should not be administered while wearing soft contact lenses.
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been conducted in animals or in humans to evaluate the possibility of these effects with fluorometholone.
Pregnancy
Pregnancy Category C
Fluorometholone has been shown to be embryocidal and teratogenic in rabbits when administered at low multiples of the human ocular dose. Fluorometholone was applied ocularly to rabbits daily on days 6-18 of gestation, and dose-related fetal loss and fetal abnormalities including cleft palate, deformed rib cage, anomalous limbs and neural abnormalities such as encephalocele, craniorachischisis, and spina bifida were observed. There are no adequate and well controlled studies of fluorometholone in pregnant women, and it is not known whether fluorometholone can cause fetal harm when administered to a pregnant woman. Fluorometholone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when FLAREX® Ophthalmic Suspension is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
ADVERSE REACTIONS
Glaucoma with optic nerve damage, visual acuity and field defects, cataract formation, secondary ocular infection following suppression of host response, and perforation of the globe may occur.
DOSAGE AND ADMINISTRATION
Shake Well Before Using. One to two drops instilled into the conjunctival sac(s) four times daily. During the initial 24 to 48 hours the dosage may be safely increased to two drops every two hours. If no improvement after two weeks, consult physician. Care should be taken not to discontinue therapy prematurely.
HOW SUPPLIED
2.5 mL, 5mL and 10 mL in plastic DROP-TAINER® dispensers.
2.5 mL: NDC 0065-0096-25
5 mL: NDC 0065-0096-05
10 mL: NDC 0065-0096-10
STORAGE: Store upright between 2°-27°C (36°-80°F). Protect From Freezing.
Rx Only
REFERENCES
- Leibowitz, H. M., et. al., Annals of Ophthalmology 1984; 16:1110.
- Stewart, R. H., et. al., Current Eye Research 1984; 3:835.
OPHTHALMIC
Alcon Laboratories, Inc.
Fort Worth , Texas 76134 USA
June 1999
340075-0699
| Flarex (fluorometholone acetate) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Revised: 03/2006Alcon Laboratories, Inc.