HAND SANITIZER
-
alcohol gel
Hanover Pen Corp dba HPC Global
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
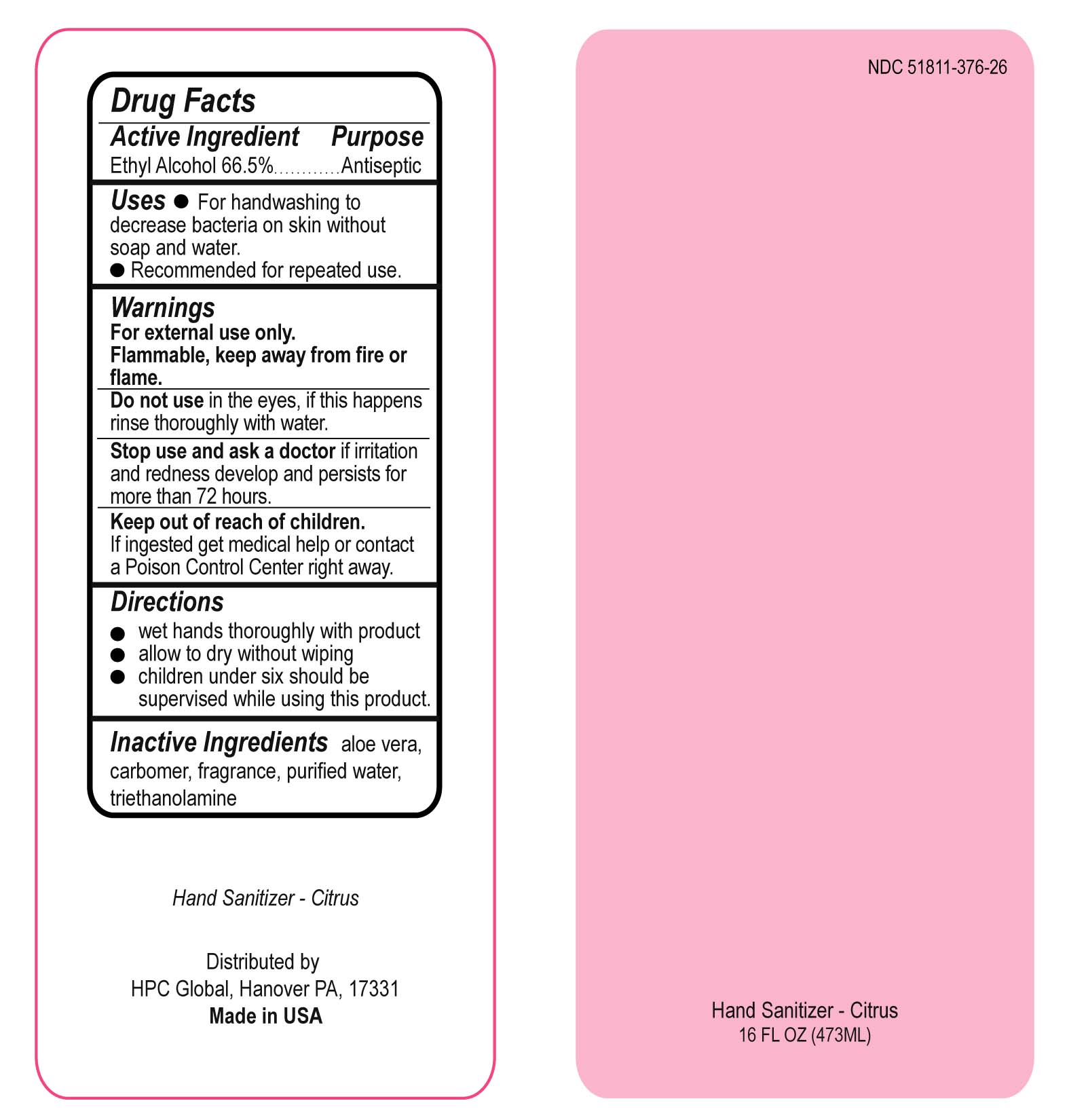
Uses
For handwashing to decrease bacteria on skin without soap and water.
Recommended for repeated use.
Stop use and ask a doctor
Stop use and ask a doctor if irritation and redness develop and persists for more than 72 hours.
Keep out of reach of children
If ingested get medical help or contact a Poison Control Center right away.
Directions
wet hands throughly with product
allow to dry without wiping
children under six should be supervised while using this produce.
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
Front Label
NDC 51811-376-26
Hand Sanitizer - Fresh
16 FL OZ (473ML)
Back Label (Drug Facts)
Hand Sanitizer - Citrus
HPC Global, Hanover PA, 17331
Made in USA
| HAND SANITIZER
alcohol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333E | 02/01/2012 | |
| Labeler - Hanover Pen Corp dba HPC Global (003022670) |
| Registrant - Hanover PEn Corp dba HPC Global (003022670) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Hanover Pen Corp dba HPC Global | 003022670 | repack, label | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Safetec of America, Inc. | 874965262 | manufacture | |
Revised: 02/2012 Hanover Pen Corp dba HPC Global