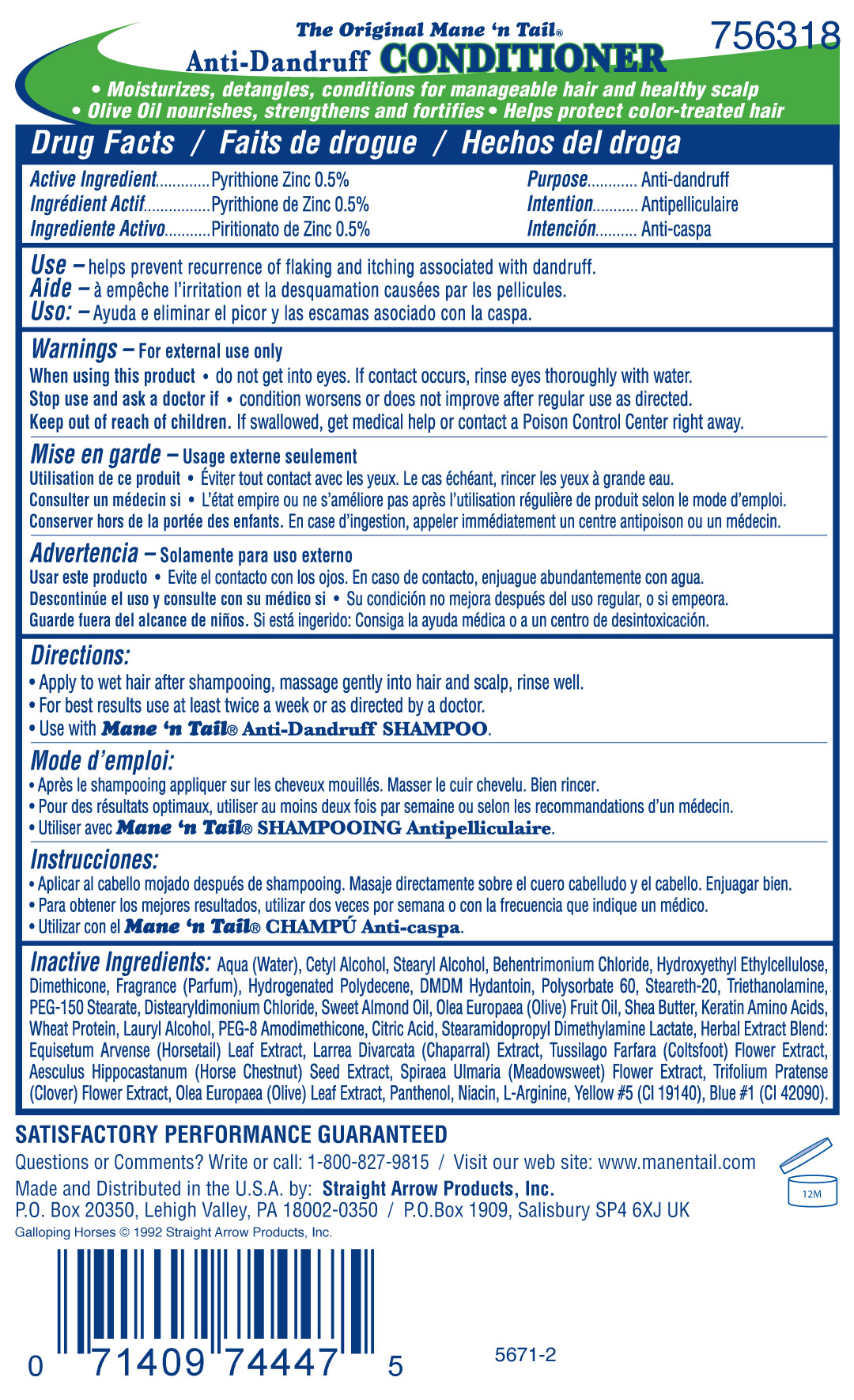
MANE N TAIL DAILY CONTROL ANTI DANDRUFF CONDITIONER - pyrithione zinc rinse
Straight Arrow Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Zinc Pyrithione 0.5%
Use -helps prevent recurrence of flaking and itching associated with dandruff.
Warnings - For external use only
When using this product
- do not get into eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- condition worsens or does not improve after regular use as directed.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
- Apply to wet hair after shampooing, massage gently into hair and scalp, rinse well.
- For best results, use at least twice a week or as directed by a doctor.
- Use with Mane 'n Tail Anti-Dandruff SHAMPOO
Inactive Ingredients: Water/Aqua/Eau, Cetyl Alcohol, Stearyl Alcohol, Behentrimonium Chloride, Hydroxyethyl Ethylcellulose, Dimethicone, Fragrance (Parfum), Hydrogenated Polydecene, DMDM Hydantoin, Polysorbate 60, Steareth-20, Triethanolamine, PEG-150 Stearate, Distearyldimonium Chloride, Prunus Amagdalus Dulcis (Sweet Almond) Seed Oil, Olea Europaea (Olive) Fruit Oil, Shea Butter, Keratin Amino Acids, Wheat Protein, Lauryl Alcohol, PEG-8 Amodimethicone, Citric Acid, Stearamidopropyl Dimethylamine Lactate, Herbal Extract Blend: Equisetum Arvense (Horsetail) Leaf Extract, Larrea Divaricata (Chaparral) Extract, Tussilago Farfara (Coltsfoot) Flower Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Spiraea Ulmaria (Meadowsweet) Flower Extract, Trifolium Pratense (Clover) Flower Extract, Panthenol, Niacin, L-Arginine, Yellow #5 (CI 19140), Blue #1 (CI 42090).
Straight Arrow Products, Inc.