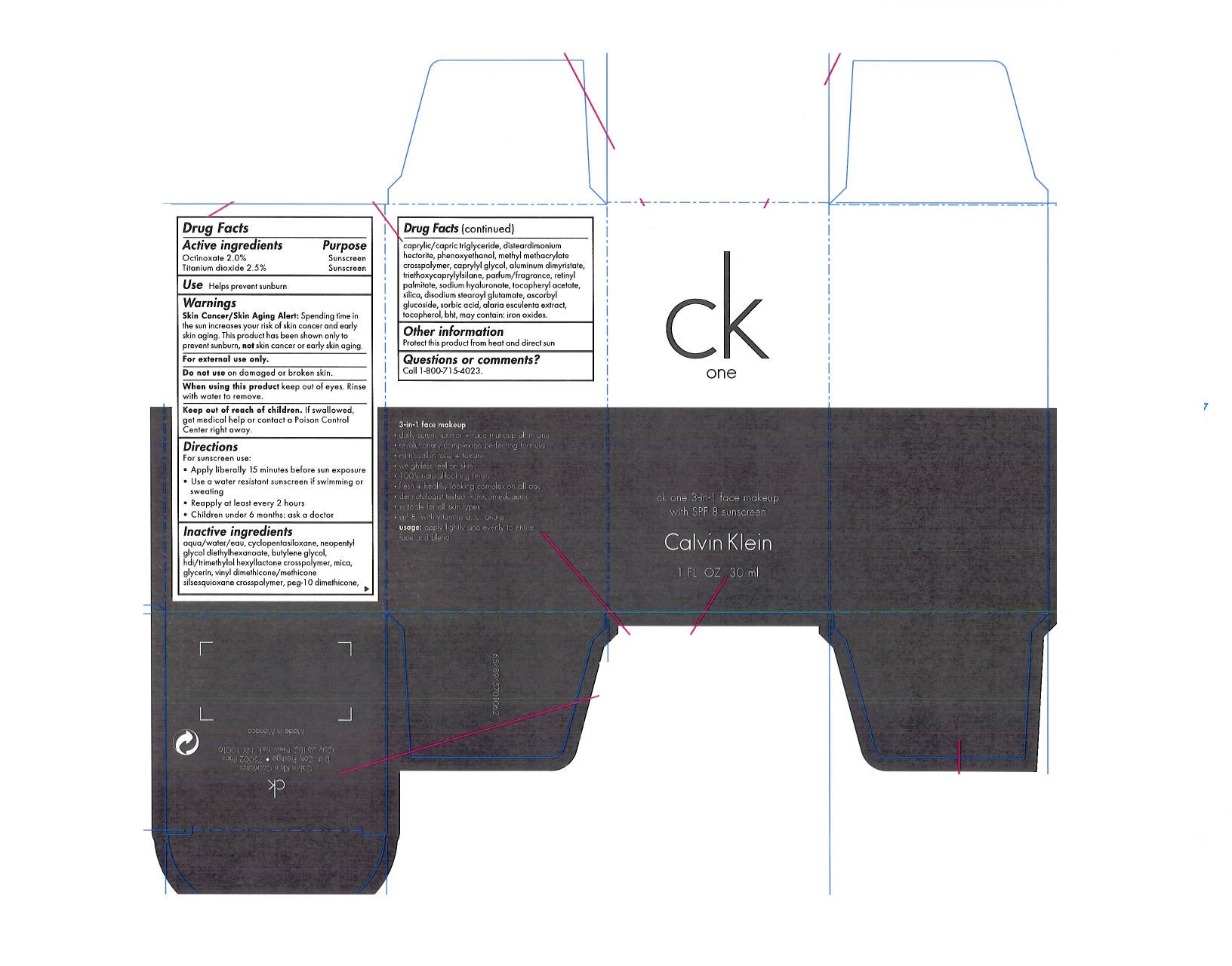
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Skin cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
water, cyclopentasiloxane, neopentyl, glycol diethylhexanoate, butylene glycol, hdi/trimethylol hexyllactone crosspolymer, mica, glycerin, vinyl dimethicone/methicone silsesquioxane crosspolymer, peg-10 dimethicone, caprylic/capric triglyceride, disteardimonium hectorite, phenoxyethanol, methyl methacrylate crosspolymer, caprylyl glycol, aluminum dimyristate, triethoxycaprylylsilane, parfum/fragrance, retynyl palmitate, sodium hyaluronate, tocopheryl acetate, silica, disodium stearoyl glutamate, ascorbyl glucoside, sorbic acid, alaria esculenta extract, tocopherol, bht, may contain: iron oxides.