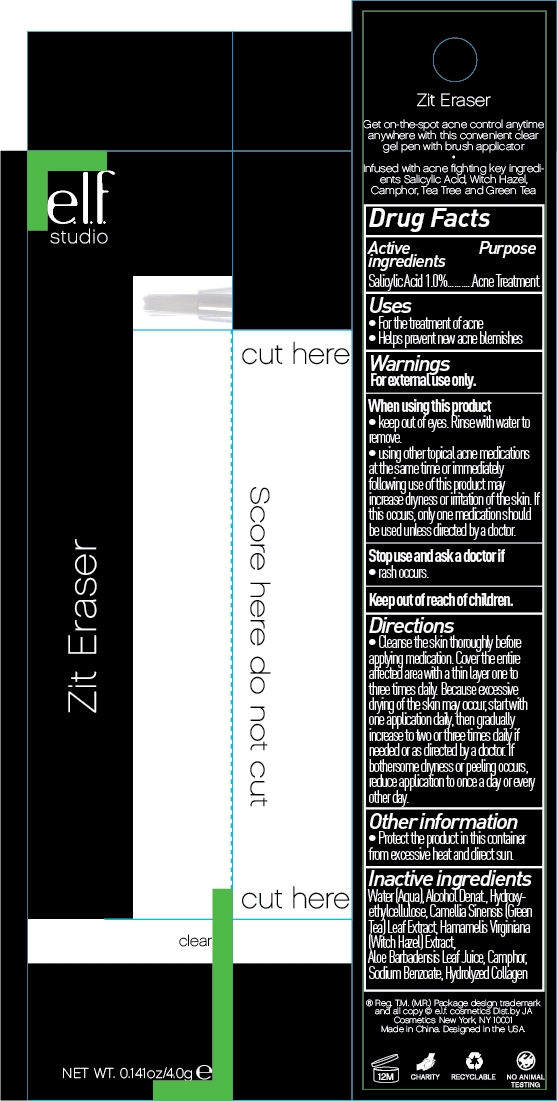
ELF ZIT ERASER
-
salicylic acid liquid
J. A. Cosmetics U.S. INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
When Using This Product:
Keep out of eyes, rinse with water to remove.
With other tropical acne medications at the same time or immediately following use of this product may increase dryness or irritation to the skin. If this occurs, only one medication should be used unless directed by your doctor.
Directions:
Cleanse the skin thoroughly before applying medication. Cover the entire affected area with a thin layer one to three times daily. Because excessive drying of skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredient:
Water (Aqua), Alcohol Denature, Hydroxyethylcellulose, Camellia Sinensis (Green Tea) Leaf Extract, Hamamelis Virginiana (Witch Hazel) Extract, Aloe Barbadensis Leaf Juice, Camphor, Sodium Benzoate, Hydrolyzed Collagen| ELF ZIT ERASER
salicylic acid liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part358H | 01/29/2012 | |
| Labeler - J. A. Cosmetics U.S. INC (186705047) |
| Registrant - J. A. Cosmetics U.S. INC (186705047) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Shanghai Justking Enterprise Co. Ltd. | 421267459 | manufacture | |
Revised: 01/2012 J. A. Cosmetics U.S. INC