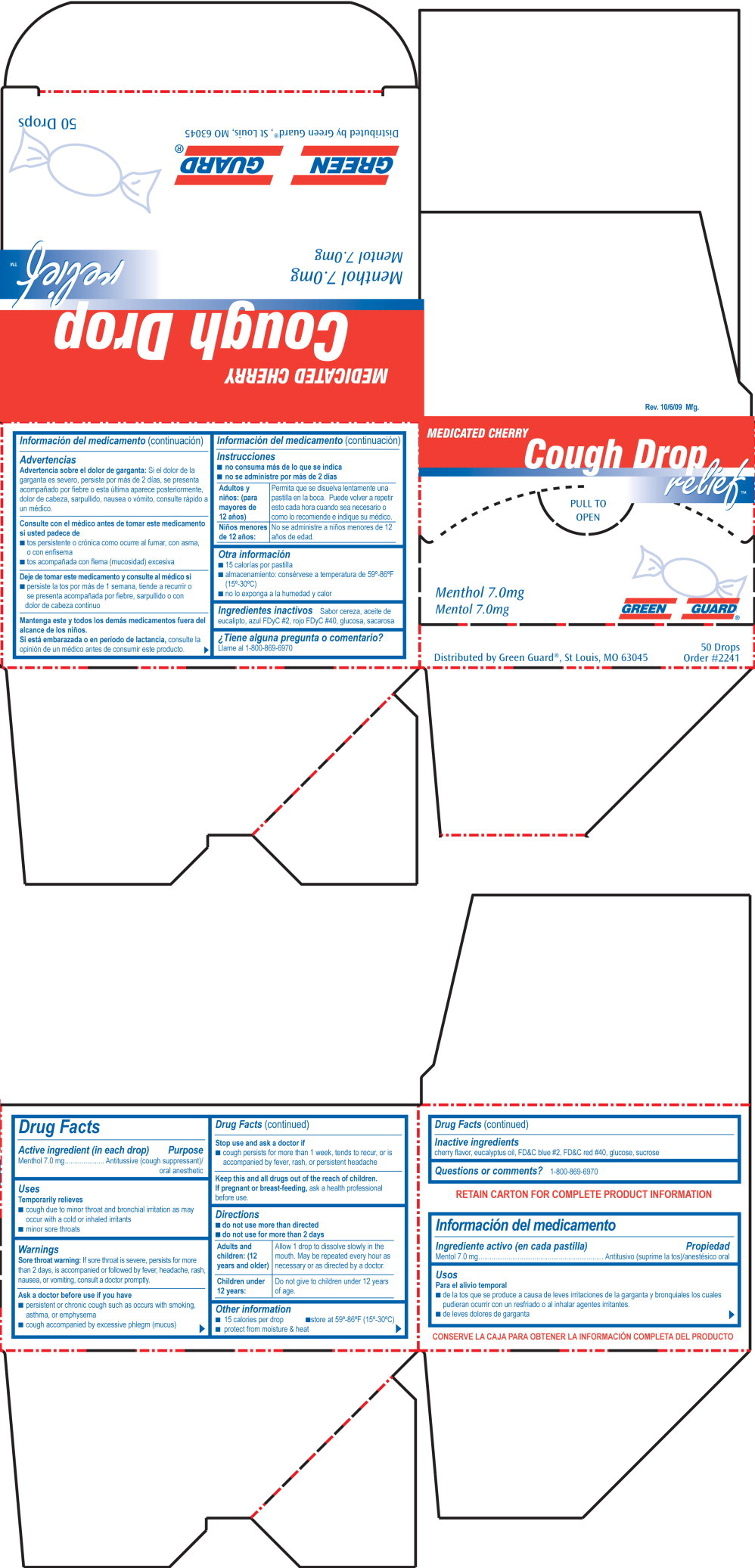
GREEN GUARD COUGH RELIEF- menthol lozenge
Unifirst First Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Green Guard Cough Relief
Warnings
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough accompanied by excessive phlegm (mucus)
| GREEN GUARD COUGH RELIEF
menthol lozenge |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Unifirst First Aid Corporation (832947092) |
Revised: 1/2012
Document Id: c05a6ede-d004-4cdb-bb07-7d586b0af5a3
Set id: 5a4522df-75d6-42a9-9571-30ecfbb60b2e
Version: 2
Effective Time: 20120127
Unifirst First Aid Corporation