MEDIQUE MEDI-PHENYL
-
phenylephrine hydrochloride tablet, film coated
DOVER SUDANYL PE
-
phenylephrine hydrochloride tablet, film coated
Unifirst First Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
Temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies.
Promotes nasal and/or sinus drainage; temporarily relieves sinus congestion and pressure.
Do not use
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
- do not exceed recommended dosage
- if nervousness, dizziness, or sleeplessness occur, discontinue use and consult a doctor
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Adults and children: (12 years and older) Take 2 tablets every 4 hours. Do not take more than 12 tablets in 24 hours.
Other information
- read all product information before using.
- contains FD&C yellow #6 (tartrazine)
- store at room temperature 59º-86º F (15º-30º C).
- tamper evident sealed packets.
- do not use any opened or torn packets
Inactive ingredients
colloidal silica*, croscarmellose sodium, D&C Red #27*, dicalcium phosphate*, FD&C red #40*, FD&C yellow #6*, hypromellose*, lactose*, magnesium stearate,
microcrystalline cellulose, pharmaceutical glaze*, polyethylene glycol*, pregelatinized starch*, silicon dioxide*, stearic acid*, talc*, titanium dioxide*.
*may contain
205R Medique Medi-Phenyl Label
Collect Medi-Bucks
See inside flap for further details
Medique®
Medi-Phenyl
Phenylephrine 5 mg
Nasal Decongestant
Descongestivo Nasal
Easy To Swallow
Film Coated Tablets
Facil de Tragar Tabletas con Cubierta Pelicular
Pull to Open
TiraParaAbrir
500 Tablets
(250 x 2)
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Untarias
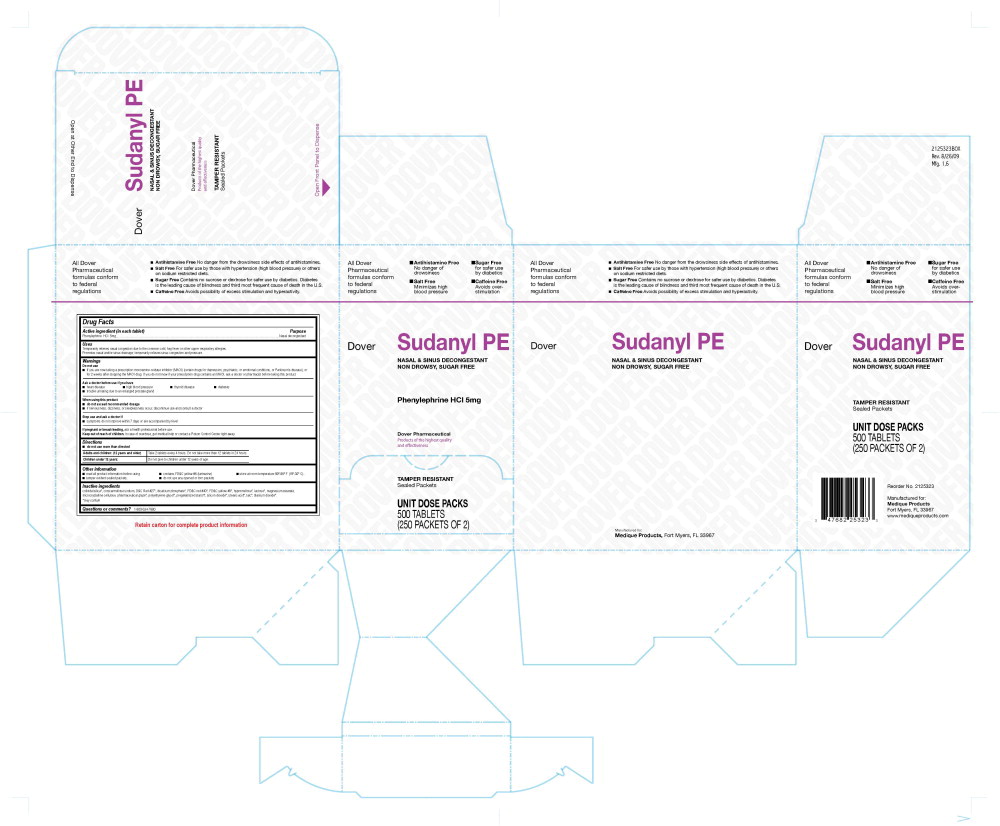
205R Dover Sudanyl Label
Dover
Sudanyl PE
Nasal and Nasal Decongestant
Non-Drowsy, Sugar Free
Phenylephrine HCl 5 mg
Dover Pharmaceutical
Products of the highest quality and effectiveness
Tamper Resistant
Sealed Packets
Unit Dose Packs
500 Tablets
(250 Packets of 2)
| MEDIQUE MEDI-PHENYL
phenylephrine hydrochloride tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | 09/21/2010 |
| DOVER SUDANYL PE
phenylephrine hydrochloride tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | 09/21/2010 |
| Labeler - Unifirst First Aid Corporation (832947092) |
Revised: 01/2012 Unifirst First Aid Corporation