LUDIOMIL
-
maprotiline hydrochloride tablet
Novartis Pharmaceuticals Corporation
----------
DESCRIPTION
Ludiomil, maprotiline hydrochloride USP, is a tetracyclic antidepressant, available as 25-mg, 50-mg and 75-mg tablets for oral administration. Its chemical name is N-methyl-9,10-ethanoanthracene-9 (10H)-propylamine hydrochloride, and its structural formula is
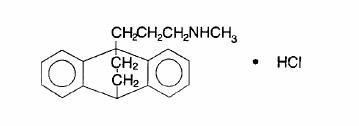
Maprotiline hydrochloride USP is a fine, white to off-white, practically odorless crystalline powder. It is freely soluble in methanol and in chloroform, slightly soluble in water, and practically insoluble in isooctane. Its molecular weight is 313.87.
Inactive Ingredients. Calcium phosphate, cellulose compounds, colloidal silicon dioxide, FD&C Yellow No. 6 Aluminum Lake (25-mg and 50-mg tablets), lactose, magnesium stearate, stearic acid, talc, titanium dioxide, polyethylene glycol, polysorbate, crospovidone, and iron oxide (75-mg tablets).
CLINICAL PHARMACOLOGY
The mechanism of action of Ludiomil is not precisely known. It does not act primarily by stimulation of the central nervous system and is not a monoamine oxidase inhibitor. The postulated mechanism of Ludiomil is that it acts primarily by potentiation of central adrenergic synapses by blocking reuptake of norepinephrine at nerve endings. This pharmacologic action is thought to be responsible for the drug's antidepressant and anxiolytic effects.
The mean time to peak is 12 hours. The half-life of elimination averages 51 hours.
Steady-state levels measured prior to the morning dose on a one-dosage regimen are summarized as follows:
| Regimen | Average Minimum Concentration ng/ml | 95% Confidence Limits ng/mL |
| 50 mg x 3 daily | 238 | 181—295 |
INDICATIONS AND USAGE
Ludiomil is indicated for the treatment of depressive illness in patients with depressive neurosis (dysthymic disorder) and manic-depressive illness, depressed type (major depressive disorder). Ludiomil is also effective for the relief of anxiety associated with depression.
CONTRAINDICATIONS
Ludiomil is contraindicated in patients hypersensitive to Ludiomil and in patients with known or suspected seizure disorders. It should not be given concomitantly with monoamine oxidase (MAO) inhibitors. A minimum of 14 days should be allowed to elapse after discontinuation of MAO inhibitors before treatment with Ludiomil is initiated. Effects should be monitored with gradual increase in dosage until optimum response is achieved. The drug is not recommended for use during the acute phase of myocardial infarction.
WARNINGS
Seizures have been associated with the use of Ludiomil.
Most of the seizures have occurred in patients without a known history of seizures. However, in some of these cases, other confounding factors were present, including concomitant medications known to lower the seizure threshold, rapid escalation of the dosage of Ludiomil, and dosage that exceeded the recommended therapeutic range. The incidence of direct reports is less than 1/10 of 1%. The risk of seizures may be increased when Ludiomil is taken concomitantly with phenothiazines, when the dosage of benzodiazepines is rapidly tapered in patients receiving Ludiomil or when the recommended dosage of Ludiomil is exceeded. While a cause-and-effect relationship has not been established, the risk of seizures in patients treated with Ludiomil may be reduced by (1) initiating therapy at a low dosage, (2) maintaining the initial dosage for 2 weeks before raising it gradually in small increments as necessitated by the long half-life of Ludiomil (average 51 hours), and (3) keeping the dosage at the minimally effective level during maintenance therapy. (See DOSAGE AND ADMINISTRATION.)
Extreme caution should be used when this drug is given to:
- patients with a history of myocardial infarction;
- patients with a history or presence of cardiovascular disease because of the possibility of conduction defects, arrhythmias, myocardial infarction, strokes and tachycardia.
PRECAUTIONS
General
The possibility of suicide in seriously depressed patients is inherent in their illness and may persist until significant remission occurs. Therefore, patients must be carefully supervised during all phases of treatment with Ludiomil, and prescriptions should be written for the smallest number of tablets consistent with good patient management.
Hypomanic or manic episodes have been known to occur in some patients taking tricyclic antidepressant drugs, particularly in patients with cyclic disorders. Such occurrences have also been noted, rarely, with Ludiomil.
Prior to elective surgery, Ludiomil should be discontinued for as long as clinically feasible, since little is known about the interaction between Ludiomil and general anesthetics.
Ludiomil should be administered with caution in patients with increased intraocular pressure, history of urinary retention, or history of narrow-angle glaucoma because of the drug's anticholinergic properties.
Information for Patients
Patients should be warned of the association between seizures and the use of Ludiomil. Moreover, they should be informed that this association is enhanced in patients with a known history of seizures and in those patients who are taking certain other drugs. (See WARNINGS.)
Warn patients to exercise caution about potentially hazardous tasks, or operating automobiles or machinery since the drug may impair mental and/or physical abilities.
Ludiomil may enhance the response to alcohol, barbiturates, and other CNS depressants, requiring appropriate caution of administration.
Laboratory Tests
Ludiomil should be discontinued if there is evidence of pathological neutrophil depression. Leukocyte and differential counts should be performed in patients who develop fever and sore throat during therapy.
Drug Interactions
Close supervision and careful adjustment of dosage are required when administering Ludiomil concomitantly with anticholinergic or sympathomimetic drugs because of the possibility of additive atropine-like effects.
Concurrent administration of Ludiomil with electroshock therapy should be avoided because of the lack of experience in this area.
Caution should be exercised when administering Ludiomil to hyperthyroid patients or those on thyroid medication because of the possibility of enhanced potential for cardiovascular toxicity of Ludiomil.
Ludiomil should be used with caution in patients receiving guanethidine or similar agents since it may block the pharmacologic effects of these drugs.
The risk of seizures may be increased when Ludiomil is taken concomitantly with phenothiazines or when the dosage of benzodiazepines is rapidly tapered in patients receiving Ludiomil.
Because of the pharmacologic similarity of Ludiomil to the tricyclic antidepressants, the plasma concentration of Ludiomil may be increased when the drug is given concomitantly with hepatic enzyme inhibitors (e.g., cimetidine, fluoxetine) and decreased by concomitant administration with hepatic enzyme inducers (e.g., barbiturates, phenytoin), as has occurred with tricyclic antidepressants. Adjustment of the dosage of Ludiomil may therefore be necessary in such cases.
(See PRECAUTIONS, Information for Patients.)
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and chronic toxicity studies have been conducted in laboratory rats and dogs. No drug- or dose-related occurrence of carcinogenesis was evident in rats receiving daily oral doses up to 60 mg/kg of Ludiomil for eighteen months or in dogs receiving daily oral doses up to 30 mg/kg of Ludiomil for one year. In addition, no evidence of mutagenic activity was found in offspring of female mice mated with males treated with up to 60 times the maximum daily human dose.
Pregnancy Category B
Reproduction studies have been performed in female laboratory rabbits, mice, and rats at doses up to 1.3, 7, and 9 times the maximum daily human dose respectively and have revealed no evidence of impaired fertility or harm to the fetus due to Ludiomil. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
Although the effect of Ludiomil on labor and delivery is unknown, caution should be exercised as with any drug with CNS depressant action.
ADVERSE REACTIONS
The following adverse reactions have been noted with Ludiomil and are generally similar to those observed with tricyclic antidepressants.
Cardiovascular
Rare occurrences of hypotension, hypertension, tachycardia, palpitation, arrhythmia, heart block, and syncope have been reported with Ludiomil.
Psychiatric
Nervousness (6%), anxiety (3%), insomnia (2%), and agitation (2%); rarely, confusional states (especially in the elderly), hallucinations, disorientation, delusions, restlessness, nightmares, hypomania, mania, exacerbation of psychosis, decrease in memory, and feelings of unreality.
Neurological
Drowsiness (16%), dizziness (8%), tremor (3%), and, rarely, numbness, tingling, motor hyperactivity, akathisia, seizures, EEG alterations, tinnitus, extrapyramidal symptoms, ataxia, and dysarthria.
Anticholinergic
Dry mouth (22%), constipation (6%), and blurred vision (4%); rarely, accommodation disturbances, mydriasis, urinary retention, and delayed micturition.
Allergic
Rare instances of skin rash, petechiae, itching, photosensitization, edema, and drug fever.
Gastrointestinal
Nausea (2%) and, rarely, vomiting, epigastric distress, diarrhea, bitter taste, abdominal cramps and dysphagia.
Endocrine
Rare instances of increased or decreased libido, impotence, and elevation or depression of blood sugar levels.
Other
Weakness and fatigue (4%) and headache (4%); rarely, altered liver function, jaundice, weight loss or gain, excessive perspiration, flushing, urinary frequency, increased salivation, nasal congestion, and alopecia.
Note
Although there have been only isolated reports of the following adverse reactions with Ludiomil, its pharmacologic similarity to tricyclic antidepressants requires that each reaction be considered when administering Ludiomil.
- Bone marrow depression, including agranulocytosis, eosinophilia, purpura, and thrombocytopenia, myocardial infarction, stroke, peripheral neuropathy, sublingual adenitis, black tongue, stomatitis, paralytic ileus, gynecomastia in the male, breast enlargement and galactorrhea in the female, and testicular swelling.
OVERDOSAGE
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after overdose. Therefore, hospital monitoring is required as soon as possible.
Animal Oral LD50: The oral LD50 of Ludiomil is 600-750 mg/kg in mice, 760-900 mg/kg in rats, > 1000 mg/kg in rabbits, > 300 mg/kg in cats, and > 30 mg/kg in dogs.
Manifestations
Data dealing with overdosage in humans are limited with only a few cases on record. Signs and symptoms of Ludiomil overdose are similar to those seen with tricyclic overdose. Critical manifestations of overdose include cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of toxicity. Other clinical manifestations include drowsiness, tachycardia, ataxia, vomiting, cyanosis, shock, restlessness, agitation, hyperpyrexia, muscle rigidity, athetoid movements, and mydriasis. Since congestive heart failure has been seen with overdosages of tricyclic antidepressants, it should be considered with Ludiomil overdosage.
Management
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line, and initiate gastric decontamination. A minimum of 6 hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. There are case reports of patients succumbing to fatal dysrhythmias late after tricyclic overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination
All patients suspected of overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
Cardiovascular
A maximal limb-lead QRS duration of ≥ 0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH >7.60 or a Pco2 < 20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium, or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective.
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
DOSAGE AND ADMINISTRATION
A single daily dose is an alternative to divided daily doses. Therapeutic effects are sometimes seen within 3 to 7 days, although as long as 2 to 3 weeks are usually necessary.
Initial Adult Dosage
An initial dosage of 75 mg daily is suggested for outpatients with mild-to-moderate depression. However, in some patients, particularly the elderly, an initial dosage of 25 mg daily may be used. Because of the long half-life of Ludiomil, the initial dosage should be maintained for two weeks. The dosage may then be increased gradually in 25-mg increments as required and tolerated. In most outpatients a maximum dose of 150 mg daily will result in therapeutic efficacy. It is recommended that this dose not be exceeded except in the most severely depressed patients. In such patients, dosage may be gradually increased to a maximum of 225 mg.
More severely depressed, hospitalized patients should be given an initial daily dose of 100 mg to 150 mg which may be gradually increased as required and tolerated. Most hospitalized patients with moderate-to-severe depression respond to a daily dose of 150 mg although dosages as high as 225 mg may be required in some cases. Daily dosage of 225 mg should not be exceeded.
HOW SUPPLIED
Tablets 25 mg – oval, dark orange, scored, coated (imprinted CIBA 110)
Bottles of 100……………………………………………………...NDC 0083-0110-30
Tablets 50 mg – round, dark orange, scored, coated (imprinted CIBA 26)
Bottles of 100……………………………………………………...NDC 0083-0026-30
Tablets 75 mg – oval, white, scored, coated (imprinted CIBA 135)
Bottles of 100……………………………………………………...NDC 0083-0135-30
Do not store above 86°F (30°C).
Dispense in tight container (USP).
C96-65 (Rev. 11/96)
C I B A
Ciba-Geigy Corporation
Pharmaceuticals Division
Summit, New Jersey 07901
| LUDIOMIL
maprotiline hydrochloride tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017543 | 12/01/1980 | 10/30/2008 |
| LUDIOMIL
maprotiline hydrochloride tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017543 | 12/01/1980 | 10/30/2008 |
| LUDIOMIL
maprotiline hydrochloride tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017543 | 12/01/1980 | 10/30/2008 |
| Labeler - Novartis Pharmaceuticals Corporation (604159772) |
Revised: 10/2010 Novartis Pharmaceuticals Corporation
