CHLOROMYCETIN OTIC
-
chloramphenicol solution/ drops
Monarch Pharmaceuticals, Inc.
----------
WARNING
Bone marrow hypoplasia including aplastic anemia and death has been reported following local application of chloramphenicol. Chloramphenicol should not be used when less potentially dangerous agents would be expected to provide effective treatment.
Description
Each milliliter of Chloromycetin Otic contains 5 mg (0.5%) chloramphenicol in propylene glycol. Sterile.
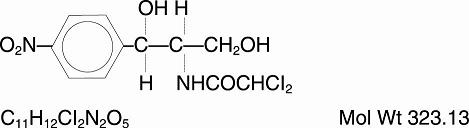
The chemical names for chloramphenicol are:
- Acetamide, 2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl) ethyl]-, and
- D-threo-(—)-2,2-Dichloro-N-[β,-hydroxy-α-(hydroxymethyl)-p-nitrophenethyl] acetamide.
Chloramphenicol has the following empirical and structural formulas:
Clinical Pharmacology
Chloramphenicol is a broad-spectrum antibiotic originally isolated from Streptomyces venezuelae. It is primarily bacteriostatic and acts by inhibition of protein synthesis by interfering with the transfer of activated amino acids from soluble RNA to ribosomes. Development of resistance to chloramphenicol can be regarded as minimal for staphylococci and many other species of bacteria.
Indications and Usage
Chloromycetin (chloramphenicol) Otic is indicated for the treatment of surface infections of the external auditory canal caused by susceptible strains of various gram-positive and gram-negative organisms including: Staphylococcus aureus, Escherichia coli, Hemophilus influenzae, Pseudomonas aeruginosa, Aerobacter aerogenes, Klebsiella pneumoniae and Proteus species.
Deeper infections should be treated with appropriate systemic antibiotics.
Contraindications
This product is contraindicated in persons sensitive to any of its components.
Perforated tympanic membrane is considered a contraindication to the use of any medication in the external ear canal.
Precautions
The prolonged use of antibiotics may occasionally result in overgrowth of nonsusceptible organisms, including fungi. If new infections appear during medication, the drug should be discontinued and appropriate measures should be taken.
In all serious infections, the topical use of chloramphenicol should be supplemented by appropriate systemic medication.
The possibility of the occurrence of ototoxicity must be considered if this product is allowed to enter the middle ear.
Adverse Reactions
Signs of local irritation with subjective symptoms of itching or burning, angioneurotic edema, urticaria, vesicular and maculopapular dermatitis have been reported in patients sensitive to chloramphenicol and are causes for discontinuing the medication. Similar sensitivity reactions to other materials in topical preparations may also occur. Blood dyscrasias have been reported in association with the use of chloramphenicol (see WARNINGS).
How Supplied
NDC 61570-331-31
Chloromycetin (chloramphenicol) Otic is supplied in 15 mL vials with droppers.
Store below 30°C (86°F).
Chloromycetin, brand of chloramphenicol, Reg US Pat Off.
Rx only.
Rev. 12/98
Manufactured by: Parkedale Pharmaceuticals, Inc., Rochester, MI 48307
Distributed by:Monarch Pharmaceuticals, Inc., Bristol, TN 37620
| CHLOROMYCETIN OTIC
chloramphenicol solution/ drops |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA050205 | 03/30/1953 | 02/12/2002 |
| Labeler - Monarch Pharmaceuticals, Inc. (809587413) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Parkedale Pharmaceuticals, Inc | 809587413 | MANUFACTURE | |
Revised: 09/2011 Monarch Pharmaceuticals, Inc.
