ATROVENT
-
ipratropium bromide aerosol, metered
Physicians Total Care, Inc.
----------
ATTENTION PHARMACISTS: Detach “Patient's Instructions for Use” and dispense with the product.
Bronchodilator Aerosol
For Oral
Inhalation Only
Prescribing Information
DESCRIPTION
The active ingredient in ATROVENT® (ipratropium bromide) Inhalation Aerosol is ipratropium bromide. It is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo[3.2.1]-octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate (endo,syn)-,(±): a synthetic quaternary ammonium compound chemically related to atropine. The structural formula is:
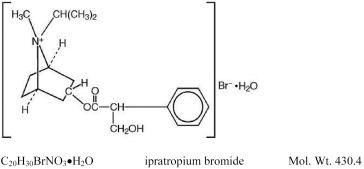
Ipratropium bromide is a white to off-white crystalline substance, freely soluble in water and lower alcohols but insoluble in lipophilic solvents such as ether, chloroform, and fluorocarbons.
ATROVENT Inhalation Aerosol contains a microcrystalline suspension of ipratropium bromide in a pressurized metered-dose aerosol unit for oral inhalation administration. The net weight is at least 14.7 grams; it yields 200 inhalations. Each actuation meters 21 mcg of ipratropium bromide from the valve and delivers 18 mcg of ipratropium bromide from the mouthpiece. The excipients are dichlorodifluoromethane, dichlorotetrafluoroethane, and trichloromonofluoromethane as propellants and soya lecithin.
CLINICAL PHARMACOLOGY
Mechanism of Action
Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally-mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released at the neuromuscular junctions in the lung. Anticholinergics prevent the increases in intracellular concentration of Ca++ which is caused by interaction of acetylcholine with the muscarinic receptors on bronchial smooth muscle.
Pharmacodynamic Properties
Controlled clinical studies have demonstrated that Atrovent® (ipratropium bromide) Inhalation Aerosol CFC does not alter either mucociliary clearance or the volume or viscosity of respiratory secretions.
Pharmacokinetics
Most of an administered dose is swallowed as shown by fecal excretion studies. Ipratropium bromide is a quaternary amine. It is not readily absorbed into the systemic circulation either from the surface of the lung or from the gastrointestinal tract as confirmed by blood level and renal excretion studies.
Autoradiographic studies in rats have shown that ipratropium bromide does not penetrate the blood-brain barrier. The half-life of elimination is about 2 hours after inhalation or intravenous administration. Ipratropium bromide is minimally bound (0 to 9% in vitro) to plasma albumin and α1-acid glycoprotein. It is partially metabolized to inactive ester hydrolysis products. Following intravenous administration, approximately one-half of the dose is excreted unchanged in the urine.
A pharmacokinetic study with 29 chronic obstructive pulmonary disease (COPD) patients (48-79 years of age) demonstrated that mean peak plasma ipratropium concentrations of 59±20 pg/mL were obtained following a single administration of 4 inhalations of ATROVENT HFA Inhalation Aerosol (84 mcg). Plasma ipratropium concentrations rapidly declined to 24±15 pg/mL by six hours. When these patients were administered 4 inhalations QID (16 inhalations/day=336 mcg) for one week, the mean peak plasma ipratropium concentration increased to 82±39 pg/mL with a trough (6 hour) concentration of 28±12 pg/mL at steady state.
Special Populations
Geriatric Patients
In the pharmacokinetic study with 29 COPD patients, a subset of 14 patients were > 65 years of age. Mean peak plasma ipratropium concentrations of 56±24 pg/mL were obtained following a single administration of 4 inhalations (21 mcg/puff) of Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol (84 mcg). When these 14 patients were administered 4 inhalations QID (16 inhalations/day) for one week, the mean peak plasma ipratropium concentration only increased to 84±50 pg/mL indicating that the pharmacokinetic behavior of ipratropium bromide in the geriatric population is consistent with younger patients.
CLINICAL PHARMACOLOGY
Mechanism of ActionIpratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released from the vagus nerve. Anticholinergics prevent the increases in intracellular concentration of cyclic guanosine monophosphate (cyclic GMP) which are caused by interaction of acetylcholine with the muscarinic receptor on bronchial smooth muscle.
PharmacokineticsThe bronchodilation following inhalation of ipratropium bromide is primarily a local, site-specific effect, not a systemic one. Much of an administered dose is swallowed as shown by fecal excretion studies. Ipratropium bromide is a quaternary amine. It is not readily absorbed into the systemic circulation either from the surface of the lung or from the gastrointestinal tract as confirmed by blood level and renal excretion studies.
The half-life of elimination is about 2 hours after inhalation or intravenous administration. Ipratropium bromide is minimally bound (0 to 9% in vitro) to plasma albumin and α1-acid glycoprotein. It is partially metabolized to inactive ester hydrolysis products. Following intravenous administration, approximately one-half of the dose is excreted unchanged in the urine. Autoradiographic studies in rats have shown that ipratropium bromide does not penetrate the blood-brain barrier.
In controlled 90 day studies in patients with bronchospasm associated with chronic obstructive pulmonary disease (chronic bronchitis and emphysema) significant improvements in pulmonary function (FEV1 and FEF25-75% increases of 15% or more) occurred within 15 minutes, reached a peak in 1-2 hours, and persisted for periods of 3 to 4 hours in the majority of patients and up to 6 hours in some patients. In addition, significant increases in Forced Vital Capacity (FVC) have been demonstrated.
Controlled clinical studies have demonstrated that ATROVENT (ipratropium bromide) Inhalation Aerosol does not alter either mucociliary clearance or the volume or viscosity of respiratory secretions. In studies without a positive control ATROVENT Inhalation Aerosol did not alter pupil size, accommodation or visual acuity (See ADVERSE REACTIONS). Ventilation/perfusion studies have shown no clinically significant effects on pulmonary gas exchange or arterial oxygen tension. At recommended doses, ATROVENT Inhalation Aerosol does not produce clinically significant changes in pulse rate or blood pressure.
INDICATIONS AND USAGE
ATROVENT (ipratropium bromide) Inhalation Aerosol is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema.
CONTRAINDICATIONS
ATROVENT (ipratropium bromide) Inhalation Aerosol is contraindicated in patients with a history of hypersensitivity to soya lecithin or related food products such as soybean and peanut. ATROVENT Inhalation Aerosol should also not be taken by patients hypersensitive to any other components of the drug product or to atropine or its derivatives.
WARNINGS
ATROVENT (ipratropium bromide) Inhalation Aerosol is not indicated for the initial treatment of acute episodes of bronchospasm where rapid response is required.
- Immediate Hypersensitivity Reactions: Immediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, anaphylaxis and oropharyngeal edema.
- Storage Conditions: The contents of ATROVENT Inhalation Aerosol are under pressure. Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120° F may cause bursting. Never throw the container into a fire or incinerator. Keep out of reach of children.
PRECAUTIONSGeneral
- Effects Seen with Anticholinergic Drugs: ATROVENT (ipratropium bromide) Inhalation Aerosol should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia or bladder-neck obstruction.
Patients should be cautioned to avoid spraying the aerosol into their eyes and be advised that this may result in precipitation or worsening of narrow-angle glaucoma, mydriasis, eye pain or discomfort, temporary blurring of vision, visual halos or colored images in association with red eyes from conjunctival and corneal congestion. Patients should also be advised that should any combination of these symptoms develop, they should consult their physician immediately.
ATROVENT Inhalation Aerosol should not be used more frequently than recommended. The dose or frequency of ATROVENT Inhalation Aerosol should not be increased without patients consulting their physician. If treatment with ATROVENT Inhalation Aerosol becomes less effective for symptomatic relief, their symptoms become worse, and/or patients need to use the product more frequently than usual, medical attention should be sought immediately. The patient, if pregnant or nursing, should be advised to contact their physician about the use of ATROVENT Inhalation Aerosol. Appropriate use of ATROVENT Inhalation Aerosol includes an understanding of the way it should be administered (See Patient's Instructions for Use).
Drug InteractionsATROVENT Inhalation Aerosol has been used concomitantly with other drugs, including sympathomimetic bronchodilators, methylxanthines, and steroids, commonly used in the treatment of chronic obstructive pulmonary disease. With the exception of albuterol, there are no formal studies fully evaluating the interaction effects of ATROVENT Inhalation Aerosol and these drugs with respect to effectiveness.
Anticholinergic agents: Although ipratropium bromide is minimally absorbed into the systemic circulation, there is some potential for an additive interaction with concomitantly used anticholinergic medications. Caution is therefore advised in the co-administration of ATROVENT Inhalation Aerosol with other anticholinergic-containing drugs.
Carcinogenesis, Mutagenesis, Impairment of FertilityTwo-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic potential at doses up to 6 mg/kg/day. This dose corresponds to approximately 360 and 180 times the maximum recommended human daily inhalation dose of ipratropium bromide in rats and mice respectively, on a mg/m2 basis. Results of various mutagenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test and chromosome aberration of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats at oral doses up to 50 mg/kg/day (approximately 3000 times the maximum recommended human daily inhalation dose on a mg/m2 basis) was unaffected by ipratropium bromide administration. At doses above 90 mg/kg/day (approximately 5400 times the maximum recommended human daily inhalation dose on a mg/m2 basis), increased resorption and decreased conception rates were observed.
PregnancyTERATOGENIC EFFECTS Pregnancy Category BOral reproduction studies were performed at doses of 10 mg/kg in mice, 100 mg/kg in rats and 125 mg/kg in rabbits. These doses correspond, in each species, respectively, to approximately 300, 6000 and 15,000 times the maximum recommended human daily inhalation dose of ipratropium bromide on a mg/m2 basis. Inhalation reproduction studies were conducted in rats and rabbits at doses of 1.5 and 1.8 mg/kg/day (approximately 90 and 210 times the maximum recommended human daily inhalation dose on a mg/m2 basis). These studies have demonstrated no evidence of teratogenic effects as a result of ipratropium bromide. However, no adequate or well controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, ATROVENT Inhalation Aerosol should be used during pregnancy only if clearly needed.
Nursing MothersIt is not known whether ATROVENT Inhalation Aerosol is excreted in human milk. Although lipid-insoluble quaternary bases pass into breast milk, it is unlikely that the active component, ipratropium bromide, would reach the infant to an important extent, especially when taken by aerosol. However, because many drugs are excreted in human milk, caution should be exercised when ATROVENT Inhalation Aerosol is administered to a nursing mother.
Pediatric UseSafety and effectiveness in the pediatric population below the age of 12 years have not been established.
PRECAUTIONS
General
ATROVENT HFA Inhalation Aerosol should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia or bladder-neck obstruction.
Information for Patients
Appropriate and safe use of ATROVENT HFA Inhalation Aerosol includes providing the patient with the information listed below and an understanding of the way it should be administered (see Patient's Instructions for Use).
Patients should be advised that ATROVENT HFA Inhalation Aerosol is a bronchodilator for the maintenance treatment of bronchospasm associated with COPD and is not indicated for the initial treatment of acute episodes of bronchospasm where rescue therapy is required for rapid response.
Patients should be cautioned to avoid spraying the aerosol into their eyes and be advised that this may result in precipitation or worsening of narrow-angle glaucoma, mydriasis, increased intraocular pressure, acute eye pain or discomfort, temporary blurring of vision, visual halos or colored images in association with red eyes from conjunctival and corneal congestion. Patients should also be advised that should any combination of these symptoms develop, they should consult their physician immediately.
The action of Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol should last 2-4 hours. Patients should be advised not to increase the dose or frequency of ATROVENT HFA Inhalation Aerosol without patients consulting their physician. Patients should also be advised to seek immediate medical attention if treatment with ATROVENT HFA Inhalation Aerosol becomes less effective for symptomatic relief, their symptoms become worse, and/or patients need to use the product more frequently than usual.
Patients should be advised on the use of ATROVENT HFA Inhalation Aerosol in relation to other inhaled drugs.
Patients should be reminded that ATROVENT HFA Inhalation Aerosol should be used consistently as prescribed throughout the course of therapy.
Patients should be advised that although the taste and inhalation sensation of ATROVENT HFA Inhalation Aerosol may be slightly different from that of the CFC (chlorofluorocarbon) formulation of ATROVENT Inhalation Aerosol, they are comparable in terms of safety and efficacy.
Since dizziness, accommodation disorder, mydriasis, and blurred vision may occur with use of ATROVENT, patients should be cautioned about engaging in activities requiring balance and visual acuity such as driving a car or operating appliances, machinery, etc.
Drug Interactions
ATROVENT HFA Inhalation Aerosol has been used concomitantly with other drugs, including sympathomimetic bronchodilators, methylxanthines, oral and inhaled steroids, that may be used in the treatment of chronic obstructive pulmonary disease. With the exception of albuterol, there are no formal studies fully evaluating the interaction effects of ATROVENT and these drugs with respect to effectiveness.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic activity at doses up to 6 mg/kg (approximately 240 and 120 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). Results of various mutagenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test and chromosome aberration of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats at oral doses up to 50 mg/kg (approximately 2000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis) was unaffected by ipratropium bromide administration. At an oral dose of 500 mg/kg (approximately 20,000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis), ipratropium bromide produced a decrease in the conception rate.
Pregnancy
Teratogenic Effects: Pregnancy Category B.
Oral reproduction studies were performed at doses of 10 mg/kg/day in mice, 1,000 mg/kg in rats and 125 mg/kg/day in rabbits. These doses correspond in each species, respectively, to approximately 200, 40000, and 10000 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis. Inhalation reproduction studies were conducted in rats and rabbits at doses of 1.5 and 1.8 mg/kg (approximately 60 and 140 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). These studies demonstrated no evidence of teratogenic effects as a result of ipratropium bromide. At oral doses 90 mg/kg and above in rats (approximately 3600 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis) embryotoxicity was observed as increased resorption. This effect is not considered relevant to human use due to the large doses at which it was observed and the difference in route of administration. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Atrovent® HFA (ipratropium bromide HFA) Inhalation Aerosol should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether the active component, ipratropium bromide, is excreted in human milk. Because lipid-insoluble quaternary cations pass into breast milk, caution should be exercised when ATROVENT HFA Inhalation Aerosol is administered to a nursing mother.
Geriatric Use
In the pivotal 12-week study, both ATROVENT HFA Inhalation Aerosol and Atrovent® (ipratropium bromide) Inhalation Aerosol CFC formulations were equally effective in patients over 65 years of age and under 65 years of age.
Of the total number of subjects in clinical studies of ATROVENT HFA Inhalation Aerosol, 57% were ≥ 65 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
ADVERSE REACTIONS
Adverse reaction information concerning ATROVENT (ipratropium bromide) Inhalation Aerosol is derived from 90 day controlled clinical trials (N=254), other controlled clinical trials using recommended doses of ATROVENT Inhalation Aerosol (N=377) and an uncontrolled study (N=1924). Additional information is derived from the post-marketing experience and the published literature.
Adverse reactions occurring in greater than one percent of patients in the 90 day controlled clinical trials appear in Table 1.
|
| Percent of Patients | |
|
| Ipratropium Bromide | Metaproterenol Sulfate |
|
| N = 254 | N= 249 |
| Reaction |
|
|
| Cardiovascular |
|
|
| Palpitations | 1.8 | 1.6 |
| Central Nervous
System |
|
|
| Nervousness | 3.1 | 6.8 |
| Dizziness | 2.4 | 2.8 |
| Headache | 2.4 | 2.0 |
| Dermatological |
|
|
| Rash | 1.2 | 0.4 |
| Gastrointestinal |
|
|
| Nausea | 2.8 | 1.2 |
| Gastrointestinal distress | 2.4 | 2.8 |
| Vomiting | 0 | 1.2 |
| Musculoskeletal |
|
|
| Tremor | 0 | 2.4 |
| Ophthalmological |
|
|
| Blurred vision | 1.2 | 0.8 |
| Oro-Otolaryngeal |
|
|
| Dry mouth | 2.4 | 0.8 |
| Irritation from aerosol | 1.6 | 1.6 |
| Respiratory |
|
|
| Cough | 5.9 | 1.2 |
| Exacerbation of symptoms | 2.4 | 3.6 |
Additional adverse reactions reported in less than one percent of the patients considered possibly due to ATROVENT Inhalation Aerosol include urinary difficulty, fatigue, insomnia and hoarseness.
The large uncontrolled, open-label study included seriously ill patients. About 7% of patients treated discontinued the program because of adverse events.
Of the 2301 patients treated in the large uncontrolled study and in clinical trials other than the 90 day studies, the most common adverse reactions reported were: dryness of the oropharynx, about 5 in 100; cough, exacerbation of symptoms and irritation from aerosol, each about 3 in 100; headache, about 2 in 100; nausea, dizziness, blurred vision/difficulty in accommodation, and drying of secretions, each about 1 in 100. Less frequently reported adverse reactions that were possibly due to ATROVENT (ipratropium bromide) Inhalation Aerosol include tachycardia, paresthesia, drowsiness, coordination difficulty, itching, hives, flushing, alopecia, constipation, tremor, and mucosal ulcers.
Cases of precipitation or worsening of narrow-angle glaucoma, acute eye pain, and hypotension, have been reported.
In a 5-year placebo-controlled trial, hospitalizations for supraventricular tachycardia and atrial fibrillation occurred with an incidence rate of 0.5% in patients receiving ATROVENT Inhalation Aerosol.
Post-Marketing ExperienceAllergic-type reactions such as skin rash, angioedema of tongue, lips and face, urticaria (including giant urticaria), laryngospasm and anaphylactic reaction have been reported, with positive rechallenge in some cases. Many of the patients had a history of allergies to other drugs and/or foods, including soybean. (See CONTRAINDICATIONS).
Additionally, urinary retention, mydriasis, and bronchospasm, including paradoxical bronchospasm, have been reported during the post-marketing period.
OVERDOSAGE
Acute overdosage by inhalation is unlikely since ipratropium bromide is not well absorbed systemically after aerosol or oral administration. The oral median lethal dose of ipratropium bromide ranged between 1001 and 2010 mg/kg in mice (approximately 30,000 and 60,000 times the maximum recommended human daily inhalation dose on a mg/m2 basis, respectively); between 1667 and 4000 mg/kg in rats (approximately 100,000 and 240,000 times the maximum recommended human daily inhalation dose, respectively, on a mg/m2 basis); and between 400 and 1300 mg/kg in dogs (approximately 80,000 and 260,000 times the maximum recommended human daily inhalation dose, respectively, on a mg/m2 basis).
DOSAGE AND ADMINISTRATION
The usual starting dose of ATROVENT (ipratropium bromide) Inhalation Aerosol is two inhalations (36 mcg) four times a day. Patients may take additional inhalations as required; however, the total number of inhalations should not exceed 12 in 24 hours. It is recommended to “test-spray” three times before using for the first time and in cases where the aerosol has not been used for more than 24 hours. Avoid spraying into eyes.
HOW SUPPLIED
ATROVENT (ipratropium bromide) Inhalation Aerosol is supplied as a metered dose inhaler with a white mouthpiece which has a clear, colorless sleeve and a green protective cap. The ATROVENT Inhalation Aerosol canister is to be used with the ATROVENT Inhalation Aerosol mouthpiece only. This mouthpiece should not be used with other aerosol medications. Similarly, the canister should not be used with other mouthpieces. Each actuation meters 21 mcg of ipratropium bromide from the valve and delivers 18 mcg of ipratropium bromide from the mouthpiece. Each 14.7 gram canister provides sufficient medication for 200 inhalations (NDC 54868-1439-1).
Note: The indented statement below is required by the Federal government's Clean Air Act for all products containing or manufactured with chlorofluorocarbons (CFCs):
Warning: Contains trichloromonofluoromethane (CFC-11), dichlorodifluoromethane (CFC-12) and dichlorotetrafluoroethane (CFC-114), substances which harm public health and the environment by destroying ozone in the upper atmosphere.A notice similar to the above Warning has been placed in the information for the patient of this product under the Environmental Protection Agency's (EPA's) regulations. The patient's warning states that the patient should consult with his or her physician if there are questions about alternatives.
Store between 59°F (15°C) and 86°F (30°C). Avoid excessive humidity.
Keep out of children's reach. Shake the canister well before using. Patients should be reminded to read and follow the accompanying “Patient's Instructions for Use”, which should be dispensed with the product. For optimal results, the canister should be at room temperature before use.
Warning: Discard the canister after you have used the labeled number of inhalations. The correct amount of medication in each inhalation cannot be assured after this point.
RxOnly
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield,
CT 06877 USA
Manufactured by:
3M Pharmaceuticals
St. Paul, MN 55144 USA
Licensed from:
Boehringer Ingelheim International GmbH
© Copyright Boehringer Ingelheim
International GmbH
2002, ALL RIGHTS
RESERVED
Revised 3/27/02
10001403/US/1
10001403/01
Relabeling of "Additional Barcode Label" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
Patient's Instructions for Use
Patient's Instructions for UseAtrovent®
(ipratropium bromide)
Inhalation Aerosol
Read complete instructions carefully before using.
- Insert metal canister into clear end of mouthpiece. Make sure the canister is fully and firmly inserted into the mouthpiece. The ATROVENT® Inhalation Aerosol canister is to be used only with the ATROVENT Inhalation Aerosol mouthpiece. This mouthpiece should not be used with other aerosol medications. Similarly, the canister should not be used with other mouthpieces.
- Remove green protective cap, hold canister as illustrated in Figure 1 and shake well before each use. If the cap is not present on the mouthpiece, the mouthpiece should be inspected for the presence of foreign objects before use. For optimal results, the canister should be at room temperature before use.
- It is recommended to “test-spray” ATROVENT Inhalation Aerosol three times
before using for the first time and in cases where the aerosol has not been used
for more than 24 hours. Avoid spraying into
eyes.
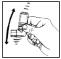
Figure 1 -
Exhale deeply through the mouth. Holding the
canister as illustrated in Figure 2, enclose
mouthpiece with the lips. Keep the eyes closed because temporary blurring of
vision, visual halos or colored images in association with red eyes from
conjunctival and corneal congestion, precipitation or worsening of narrow-angle
glaucoma, pupil dilation, or eye pain/discomfort may result if the aerosol is
sprayed into the eyes.
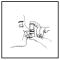
Figure 2 -
Inhale slowly through the mouth and at the same time firmly press once on the upended metal canister base as in
Figure 3; continue to inhale deeply.

Figure 3 -
Hold your breath for ten seconds and then
remove the mouthpiece from the mouth and exhale slowly, as in Figure 4. Wait approximately
fifteen seconds, shake the inhaler again and repeat previous steps 4-6.

Figure 4 - Replace protective cap after use.
- Keep the mouthpiece clean. Wash with hot water. If soap is used, rinse thoroughly with plain water. Dry thoroughly before use. When dry, replace cap on the mouthpiece when not using the drug product.
- Track the number of sprays used and discard after 200 sprays. The amount of medication in each inhalation cannot be assured after 200 sprays.
- While taking ATROVENT Inhalation Aerosol, other inhaled drugs should be taken only as directed by your physician.
- If the recommended dosage does not provide relief or symptoms become worse, patients should seek immediate medical attention. Do not increase the dose or frequency of ATROVENT Inhalation Aerosol without consulting your physician.
-
Note: The indented statement below is required by
the Federal government's Clean Air Act for all products containing or
manufactured with chlorofluorocarbons (CFCs):
This product contains trichloromonofluoromethane (CFC-11),
dichlorodifluoromethane (CFC-12) and dichlorotetrafluoroethane (CFC-114),
substances which harm the environment by destroying ozone in the upper
atmosphere.
Your physician has determined that this product is likely to help your personal health. USE THIS PRODUCT AS DIRECTED, UNLESS INSTRUCTED TO DO OTHERWISE BY YOUR PHYSICIAN. If you have any question about alternatives, consult with your physician.
- The contents of ATROVENT Inhalation Aerosol are under pressure. Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 120°F may cause bursting. Never throw the container into a fire or incinerator. Keep out of reach of children. Avoid spraying into eyes.
Store between 59°F (15°C) and 86°F (30°C). Avoid excessive humidity.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield,
CT 06877 USA
Manufactured by:
3M Pharmaceuticals
St. Paul, MN 55144 USA
Licensed from:
Boehringer Ingelheim International GmbH
© Copyright Boehringer Ingelheim
International GmbH
2002, ALL RIGHTS
RESERVED
10001403/US/1
10001403/01
| ATROVENT
ipratropium bromide monhydrate aerosol, metered |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019085 | 08/07/1995 | 01/20/2006 |
| Labeler - Physicians Total Care, Inc. (194123980) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Physicians Total Care, Inc. | 194123980 | relabel | |
Revised: 11/2010 Physicians Total Care, Inc.
