ISMELIN
-
guanethidine monosulfate tablet
Novartis Pharmaceuticals Corporation
----------
DESCRIPTION
Ismelin, guanethidine monosulfate USP, is an antihypertensive, available as tablets of 10 mg and 25 mg for oral administration. Each 10-mg and 25-mg tablet contains guanethidine monosulfate USP equivalent to 10 mg and 25 mg of guanethidine sulfate USP. Its chemical name is [2-(hexahydro-1(2H)-azocinyl)ethyl]guanidine sulfate 1:1, and its structural formula is
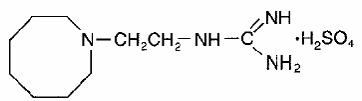
Guanethidine monosulfate USP is a white to off-white crystalline powder with a molecular weight of 296.38. It is very soluble in water, sparingly soluble in alcohol, and practically insoluble in chloroform.
Inactive Ingredients. Calcium stearate, colloidal silicon dioxide, D&C Yellow No. 10 (10-mg tablets), lactose, starch, stearic acid, and sucrose.
CLINICAL PHARMACOLOGY
Ismelin acts at the sympathetic neuroeffector junction by inhibiting or interfering with the release and/or distribution of the chemical mediator (presumably the catecholamine norepinephrine), rather than acting at the effector cell by inhibiting the association of the transmitter with its receptors. In contrast to ganglionic blocking agents, Ismelin suppresses equally the responses mediated by alpha-and beta-adrenergic receptors but does not produce parasympathetic blockade. Since sympathetic blockade results in modest decreases in peripheral resistance and cardiac output, Ismelin lowers blood pressure in the supine position. It further reduces blood pressure by decreasing the degree of vasoconstriction that normally results from reflex sympathetic nervous activity upon assumption of the upright posture, thus reducing venous return and cardiac output more. The inhibition of sympathetic venoconstrictive mechanisms results in venous pooling of blood. Therefore, the effect of Ismelin is especially pronounced when the patient is standing. Both the systolic and diastolic pressures are reduced.
Other actions at the sympathetic nerve terminal include depletion of norepinephrine. Once it gains access to the neuron, Ismelin accumulates within the intraneuronal storage vesicles and causes depletion of norepinephrine stores within the nerve terminal. Prolonged oral administration of Ismelin produces a denervation sensitivity of the neuroeffector junction, probably resulting from the chronic reduction in norepinephrine released by the sympathetic nerve endings. Systemic responses to catecholamines released from the adrenal medulla are not prevented and may even be augmented as a result of this denervation sensitivity. A paradoxical hypertensive crisis may occur if Ismelin is given to patients with pheochromocytoma or if norepinephrine is given to a patient receiving the drug.
Due to its poor lipid solubility, Ismelin does not readily cross the blood-brain barrier. In contrast to most neural blocking agents, Ismelin does not appear to suppress plasma renin activity in many patients.
Pharmacokinetics
The pharmacokinetics of Ismelin are complex. The amount of drug in plasma and in urine is linearly related to dose, although large differences occur between individuals because of variation in absorption and metabolism. Adrenergic blockade occurs with a minimum concentration in plasma of 8 ng/ml; this concentration is achieved in different individuals with dosages of 10-50 mg/day at steady state. Ismelin is eliminated slowly because of extensive tissue binding. After chronic oral administration, the initial phase of elimination with a half-life of 1.5 days is followed by a second phase of elimination with a half-life of 4-8 days. The renal clearance of Ismelin is 56 ml/min. Ismelin is converted by the liver to three metabolites, which are excreted in the urine. The metabolites are pharmacologically less active than Ismelin.
INDICATIONS AND USAGE
Ismelin is indicated for the treatment of moderate and severe hypertension, either alone or as an adjunct, and for the treatment of renal hypertension, including that secondary to pyelonephritis, renal amyloidosis, and renal artery stenosis.
CONTRAINDICATIONS
Known or suspected pheochromocytoma; hypersensitivity; frank congestive heart failure not due to hypertension; use of monoamine oxidase (MAO) inhibitors.
WARNINGS
Ismelin is a potent drug and its use can lead to disturbing and serious clinical problems. Before prescribing, physicians should familiarize themselves with the details of its use and warn patients not to deviate from instructions.
| Orthostatic hypotension can occur frequently, and patients should be properly instructed about this potential hazard. Fainting spells may occur unless the patient is forewarned to sit or lie down with the onset of dizziness or weakness. Postural hypotension is most marked in the morning and is accentuated by hot weather, alcohol, or exercise. Dizziness or weakness may be particularly bothersome during the initial period of dosage adjustment and with postural changes, such as arising in the morning. The potential occurrence of these symptoms may require alteration of previous daily activity. The patient should be cautioned to avoid sudden or prolonged standing or exercise while taking the drug. |
Inhibition of ejaculation has been reported in animals (see PRECAUTIONS, Carcinogenesis, Mutagenesis, Impairment of Fertility) as well as in men given Ismelin. This effect, which results from the sympathetic blockade caused by the drug's action, is reversible after Ismelin has been discontinued for several weeks. The drug does not cause parasympathetic blockade, and erectile potency is usually retained during administration of Ismelin. The possible occurrence of inhibition of ejaculation should be kept in mind when considering the use of guanethidine in men of reproductive age.
If possible, therapy should be withdrawn 2 weeks prior to surgery to reduce the possibility of vascular collapse and cardiac arrest during anesthesia. If emergency surgery is indicated, preanesthetic and anesthetic agents should be administered cautiously in reduced dosage. Oxygen, atropine, vasopressors, and adequate solutions for volume replacement should be ready for immediate use to counteract vascular collapse in the surgical patient. Vasopressors should be used only with extreme caution, since Ismelin augments responsiveness to exogenously administered norepinephrine and vasopressors; specifically, blood pressure may rise and cardiac arrhythmias may be produced.
PRECAUTIONS
General
Dosage requirements may be reduced in the presence of fever.
Special care should be exercised when treating patients with a history of bronchial asthma; asthmatic patients are more apt to be hypersensitive to catecholamine depletion, and their condition may be aggravated.
The effects of Ismelin are cumulative over long periods; initial doses should be small and increased gradually in small increments.
Ismelin should be used very cautiously in hypertensive patients with: renal disease and nitrogen retention or rising BUN levels, since decreased blood pressure may further compromise renal function; coronary insufficiency or recent myocardial infarction; and cerebrovascular disease, especially with encephalopathy.
Ismelin should not be given to patients with severe cardiac failure except with extreme caution, since Ismelin may interfere with the compensatory role of the adrenergic system in producing circulatory adjustment in patients with congestive heart failure.
Patients with incipient cardiac decompensation should be watched for weight gain or edema, which may be averted by the concomitant administration of a thiazide.
Ismelin should be used cautiously in patients with a history of peptic ulcer or other chronic disorders that may be aggravated by a relative increase in parasympathetic tone.
Information for Patients
The patient should be advised to take Ismelin exactly as directed. If the patient misses a dose, he or she should be told to take only the next scheduled dose (without doubling it).
The patient should be advised to avoid sudden or prolonged standing or exercise and to arise slowly, especially in the morning, to reduce the orthostatic hypotensive effects of dizziness, lightheadedness, or fainting.
The patient should be cautioned about ingesting alcohol, since it aggravates the orthostatic hypotensive effects of Ismelin.
Male patients should be advised that guanethidine may interfere with ejaculation.
Drug Interactions
Concurrent use of Ismelin and rauwolfia derivatives may cause excessive postural hypotension, bradycardia, and mental depression.
Both digitalis and Ismelin slow the heart rate.
Thiazide diuretics enhance the antihypertensive action of Ismelin (see DOSAGE AND ADMINISTRATION).
Amphetamine-like compounds, stimulants (e. g., ephedrine, methylphenidate), tricyclic antidepressants (e.g., amitriptyline, imipramine, desipramine) and other psychopharmacologic agents (e.g., phenothiazines and related compounds), as well as oral contraceptives, may reduce the hypotensive effect of Ismelin.
MAO inhibitors should be discontinued for at least 1 week before starting therapy with Ismelin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in animals have not been conducted with Ismelin.
While inhibition of sperm passage and accumulation of sperm debris have been reported in rats and rabbits after several weeks of administration of Ismelin, 5 or 10 mg/kg per day, subcutaneously or intraperitoneally, recovery of ejaculatory function and fertility has been demonstrated in rats given Ismelin intramuscularly, 25 mg/kg per day, for 8 weeks. Inhibition of ejaculation has also been reported in men (see WARNINGS and ADVERSE REACTIONS). This effect, which is attributable to the sympathetic blockade caused by the drug, is reversible several weeks after discontinuance of the drug.
Pregnancy Category C
Animal reproduction studies have not been conducted with Ismelin. It is also not known whether Ismelin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Ismelin should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency. Consequently the reactions are categorized by organ system and are listed in decreasing order of severity and not frequency.
Digestive: Diarrhea, which may be severe at times and necessitate discontinuance of medication; vomiting; nausea: increased bowel movements; dry mouth: parotid tenderness.
Cardiovascular: Chest pains (angina): bradycardia; a tendency toward fluid retention and edema with occasional development of congestive heart failure
Respiratory: Dyspnea; asthma in susceptible individuals: nasal congestion.
Neurologic: Syncope resulting from either postural or exertional hypotension; dizziness; blurred vision, muscle tremor; ptosis of the lids; mental depression; chest paresthesias; weakness; lassitude; fatigue.
Muscular: Myalgia.
Genitourinary: Rise in BUN; urinary incontinence; inhibition of ejaculation; nocturia.
Metabolic: Weight gain.
Skin and Appendages: Dermatitis; scalp hair loss.
Although a causal relationship has not been established. a few instances of blood dyscrasias (anemia, thrombocytopenia, and leukopenia) and of priapism or impotence have been reported.
OVERDOSAGE
Signs and Symptoms
Postural hypotension (with dizziness, blurred vision, and possibly syncope when standing), shock, and bradycardia are most likely to occur; diarrhea (possibly severe), nausea, and vomiting may also occur. Unconsciousness is unlikely if adequate blood pressure and cerebral perfusion can be maintained by placing the patient in the supine position and by administering other treatment as required.
Treatment
There is no specific antidote.
Treatment should consist of gastric lavage. An activated charcoal slurry should be instilled and laxatives given, if conditions permit.
In sinus bradycardia, atropine should be administered.
In previously normotensive patients, treatment has consisted essentially of restoring blood pressure and heart rate to normal by keeping the patient in the supine position. Normal homeostatic control usually returns gradually over a 72-hour period in these patients.
In previously hypertensive patients, particularly those with impaired cardiac reserve or other cardiovascular- renal disease, intensive treatment may be required to support vital functions and to control cardiac irregularities that might be present. The supine position must be maintained; if vasopressors are required, they must be used with extreme caution, since Ismelin may increase responsiveness, causing a rise in blood pressure and development of cardiac arrhythmias.
Diarrhea, if severe or persistent, should be treated with anticholinergic agents to reduce intestinal hypermotility; hydration and electrolyte balance should be maintained.
Since Ismelin is excreted slowly, cardiovascular and renal function should be monitored for a few days.
DOSAGE AND ADMINISTRATION
Better control may be obtained, especially in the initial phases of treatment, if the patient can have his blood pressure recorded regularly at home.
Ambulatory Patients
Initial doses should be small (10 mg) and increased gradually, depending upon the patient's response. Ismelin has a long duration of action; therefore, dosage increases should not be made more often than every 5-7 days, unless the patient is hospitalized.
Blood pressure should be measured in the supine position, after standing for 10 minutes, and immediately after exercise if feasible. Dosage may be increased only if there has been no decrease in the standing blood pressure from previous levels. The average daily dose is 25-50 mg; only one dose a day is usually required.
Dosage Chart for Ambulatory Patients
Visits (intervals of 5 - 7 Days) Daily Dose
Visit 1 (Patient may be started on 10-mg tablets)............................................................ 10 mg
Visit 2 ...............................................................................................................................20 mg
Visit 3 (Patient may be changed to 25-mg tablets whenever convenient)....................... 30 mg (three 10-mg tablets) or 37.5 mg (one and one-half 25-mg tablets)
Visit 4 .............................................................................................................................. 50 mg
Visit 5 and subsequent........................... Dosage may be increased by 12.5 mg or 25 mg if necessary.
The dosage should be reduced in any of the following situations: (1) normal supine pressure: (2) excessive orthostatic fall in pressure; (3) severe diarrhea.
Hospitalized Patients
Initial oral dose is 25-50 mg, increased by 25 mg or 50 mg daily or every other day, as indicated. This higher dosage is possible because hospitalized patients can be watched carefully. Unless absolutely impossible, the standing blood pressure should be measured regularly. Patients should not be discharged from the hospital until the effect of the drug on the standing blood pressure is known. Patients should be told about the possibility of orthostatic hypotension and warned not to get out of bed without help during the period of dosage adjustment.
Combination Therapy
Ismelin may be added gradually to thiazides and/or hydralazine. Thiazide diuretics enhance the effectiveness of Ismelin and may reduce the incidence of edema. When thiazide diuretics are added to the regimen in patients taking Ismelin, it is usually necessary to reduce the dosage of Ismelin. After control is established, the dosage of all drugs should be reduced to the lowest effective level.
Note: When Ismelin is replacing MAO inhibitors, at least 1 week should elapse before commencing treatment with Ismelin (see CONTRAINDICATIONS). If ganglionic blockers have not been discontinued before Ismelin is started, they should be gradually withdrawn to prevent a spiking blood pressure response during the transfer period.
HOW SUPPLIED
Tablets 10 mg – round, pale yellow, scored (imprinted CIBA 49)
Bottles of 100..................................................................................NDC 0083-0049-30
Tablets 25 mg – round, white, scored (imprinted CIBA 103)
Bottles of 100..................................................................................NDC 0083-0103-30
Do not store above 86°F (30°C).
Dispense in tight container (USP).
C96-14(Rev.3/96)
C I B A
Ciba-Geigy Corporation
Pharmaceuticals Division
Summit, New Jersey 07901
| ISMELIN
guanethidine monosulfate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA012329 | 07/05/1960 | 07/31/2008 |
| ISMELIN
guanethidine monosulfate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA012329 | 07/05/1960 | 07/31/2008 |
| Labeler - Novartis Pharmaceuticals Corporation (604159772) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Novartis Pharmaceuticals Corporation (Suffern) | 013238480 | MANUFACTURE | |
Revised: 10/2010 Novartis Pharmaceuticals Corporation
