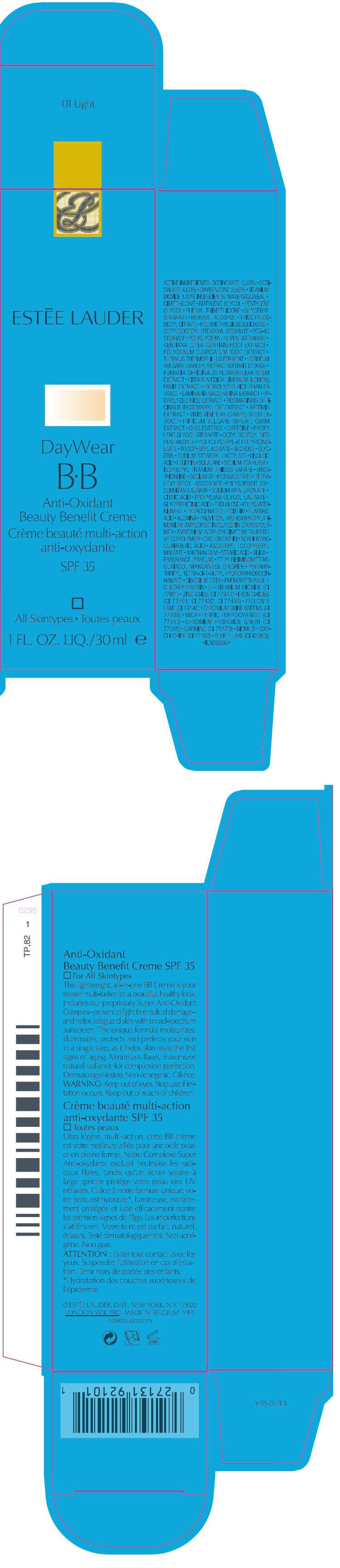
DAYWEAR BB ANTI OXIDANT BEAUTY BENEFIT SPF 35
-
octinoxate,
octisalate,
oxybenzone and
titanium dioxide cream
ESTEE LAUDER INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENTS
OCTINOXATE 7.50% ∙ OCTISALATE 4.00% ∙ OXYBENZONE 2.50% ∙ TITANIUM DIOXIDE 1.10%
INGREDIENTS
WATER • DIMETHICONE • BUTYLENE GLYCOL • PENTYLENE GLYCOL • PHENYL TRIMETHICONE • GLYCERYL STEARATE • BEHENYL ALCOHOL • TRIOCTYLDODECYL CITRATE • POLYMETHYLSILSESQUIOXANE • OCTYLDODECYL STEAROYL STEARATE • PEG-40 STEARATE • POLYGLYCERYL-10 PENTASTEARATE • GENTIANA LUTEA (GENTIAN) ROOT EXTRACT • POLYGONUM CUSPIDATUM ROOT EXTRACT • THERMUS THERMOPHILLUS FERMENT • HORDEUM VULGARE (BARLEY) EXTRACT\EXTRAIT D'ORGE • FUMARIA OFFICINALIS FLOWER/LEAF/STEM EXTRACT • CITRUS MEDICA LIMONUM (LEMON) FRUIT EXTRACT • HYDROLYZED RICE BRAN EXTRACT • LAMINARIA SACCHARINA EXTRACT • HYDROLYZED RICE EXTRACT • ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT • ARTEMIA EXTRACT • VITIS VINIFERA (GRAPE) SEED EXTRACT • TRITICUM VULGARE (WHEAT) GERM EXTRACT • CHOLESTEROL • CAFFEINE • PROPY LENE GLYCOL STEARATE • OCTYLDODECYL NEOPENTANOATE • POLYGLYCERYL-6 POLYRICINOLEATE • TOCOPHERYL ACETATE • SUCROSE • GLYCERINE • SODIUM STEAROYL LACTYLATE • LINOLEIC ACID • LECITHIN • SQUALANE • SODIUM PCA • UREA • ISOPROPYL TITANIUM TRIISOSTEARATE • ERGOTHIONEINE • SODIUM DEHYDROACETATE • TETRAHEXYLDECYL ASCORBATE • POLYSORBATE 20 • SORBITAN LAURATE • SODIUM HYALURONATE • CITRIC ACID • PROPYLENE GLYCOL LAURATE • GLYCYRRHETINIC ACID • TREHALOSE • POLYQUATERNIUM-51 • HYDROGENATED LECITHIN • FUMARIC ACID • ALUMINA • PALMITOYL HYDROXYPROPYLTRIMONIUM AMYLOPECTIN/GLYCERIN CROSSPOLYMER • AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER • CYCLODEXTRIN • NORDIHYDROGUAIARETIC ACID • ASCORBYL TOCOPHERYL MALEATE • XANTHAN GUM • STEARIC ACID • SILICA • FRAGRANCE • ETHYLBISIMINOMETHYLGUAIACOL MANGANESE CHLORIDE • PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE • DISODIUM EDTA • PHENOXYETHANOL • CHLORPHENESIN • [+/- TITANIUM DIOXIDE (CI 77891) • ZINC OXIDE (CI 77947) • IRON OXIDES (CI 77491, CI 77492, CI 77499) • YELLOW 5 LAKE (CI 19140) • CHROMIUM OXIDE GREENS (CI 77288) • MICA • FERRIC FERROCYANIDE (CI 77510) • CHROMIUM HYDROXIDE GREEN (CI 77289) • CARMINE (CI 75470) • BISMUTH OXYCHLORIDE (CI 77163) • BLUE 1 LAKE (CI 42090)] <ILN38365>
WARNING
Keep out of eyes. Stop use if irritation occurs. Keep out of reach of children.
© ESTĒE LAUDER, DIST., NEW YORK, N.Y. 10022
PRINCIPAL DISPLAY PANEL - 30 ml carton
ESTĒE LAUDER
DayWear
B•B
Anti-Oxidant
Beauty Benefit Creme
SPF 35
All Skintypes
1 FL. OZ. LIQ./30 ml e
DAYWEAR BB
ANTI OXIDANT BEAUTY BENEFIT SPF 35
octinoxate, octisalate, oxybenzone, and titanium dioxide cream |
|
|
|
|
|
|
|
|
|
|
Revised: 12/2011 ESTEE LAUDER INC