HELIUM
-
helium gas
Linde LLC
----------
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
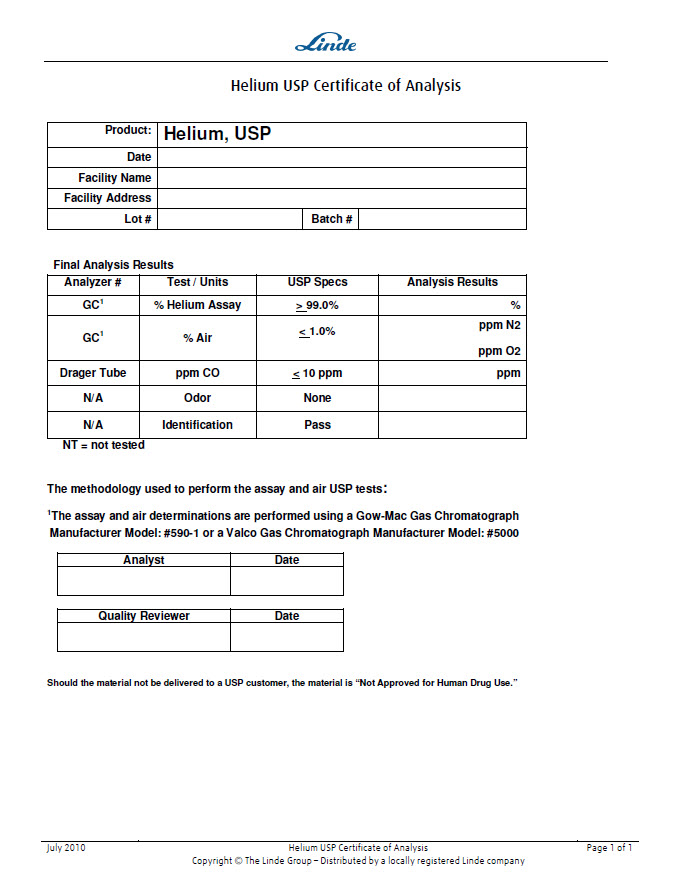
Helium USP Certificate of Analysis
|
Product: | Helium, USP | ||
|
Date |
|
||
|
Facility Name |
|
||
|
Facility Address |
|
||
|
Lot # |
|
Batch # |
|
Final Analysis Results
|
Analyzer # |
Test / Units |
USP Specs |
Analysis Results |
|
GC1 |
% Helium Assay |
> 99.0% |
% |
|
GC1 |
% Air |
< 1.0%
|
ppm N2
ppm O2 |
|
Drager Tube |
ppm CO |
< 10 ppm |
ppm |
|
N/A |
Odor |
None |
|
|
N/A |
Identification |
Pass |
|
NT = not tested The methodology used to perform the assay and air USP tests:1The assay and air determinations are performed using a Gow-Mac Gas Chromatograph Manufacturer Model: #590-1 or a Valco Gas Chromatograph Manufacturer Model: #5000
|
Analyst |
Date |
|
|
|
|
Quality Reviewer |
Date |
|
|
|
Should the material not be delivered to a USP customer, the material is “Not Approved for Human Drug Use.”
July 2010
Helium USP Certificate of Analysis
Page 1 of 1
Copyright © The Linde Group – Distributed by a locally registered Linde company
| HELIUM
helium gas |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved medical gas | 08/26/1965 | 12/15/2011 | |
| Labeler - Linde LLC (001368141) |
Revised: 12/2011 Linde LLC