dextrose (Dextrose monohydrate) injection, solution
[Baxter Healthcare Corporation ]
DESCRIPTION
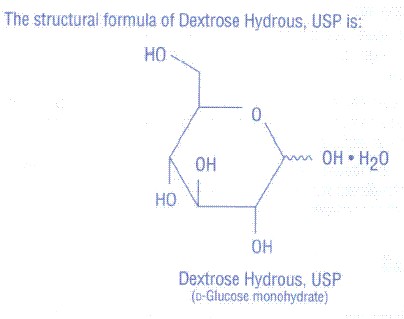
Dextrose Injections, USP are sterile, nonpyrogenic hypertonic solutions for fluid replenishment and caloric supply in Pharmacy Bulk Package. A Pharmacy Bulk Package is a container of sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion. They contain no antimicrobial agents. Composition, osmolarity, pH, and caloric content are shown below.
| Table 1. | |||||
| Composition | Osmolarity
(mOsmol/L) (calc.) | pH | Caloric
Content
(kcal/L) | How Supplied | |
| Dextrose Hydrous, USP (g/L) | Size, code, NDC | ||||
| 2000 mL unit | |||||
| 50% Dextrose Injection, USP | 500 | 2520 |
4.0 (3.2 to 6.5) | 1710 |
2B0256 NDC 0338-0031-06 |
| 70% Dextose Injection, USP | 700 | 3530 |
4.0 (3.2 to 6.5) | 2390 |
2B0296 NDC 0338-0719-06 |
The VIAFLEX plastic container is fabricated from a specially formulated polyvinyl choloride (PL 146 Plastic). Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly.
Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as tissue culture toxicity studies.
CLINICAL PHARMACOLOGY
Dextrose Injections, USP have value as a source of water and calories. They are capable of inducing diuresis depending on the clinical condition of the patient.
INDICATIONS AND USAGE
Dextrose Injections, USP are indicated as a caloric component in a parenteral nutrition regimen. They are used with an appropriate protein (nitrogen) source in the prevention of nitrogen loss or in the treatment of negative nitrogen balance in patients where: (1) the alimentary tract cannot or should not be used, (2) gastrointestinal absorption of protein is impaired, or (3) metabolic requirements for protein are substantially increased, as with extensive burns
CONTRAINDICATIONS
The infusion of hypertonic dextrose injections is contraindicated in patients having intracranial or intraspinal hemorrhage, in patients who are severely dehydrated, in patients who are anuric, and in patients in hepatic coma.
Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
WARNINGS
These injections are for compounding only, not for direct infusion.
Dilute before use to a concentration which will, when administered with an amino acid (nitrogen) source, result in an appropriate calorie to gram of nitrogen ratio and which has an osmolarity consistent with the route of administration.
Unless appropriately diluted, the infusion of hypertonic dextrose injection into a peripheral vein may result in vein irritation, vein damage, and thrombosis. Strongly hypertonic nutrient solutions should only be administered through an indwelling intravenous catheter with the tip located in a large central vein such as the superior vena cava.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 µg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Administration by central venous catheter should be used only by those familiar with this technique and its complications.
PRECAUTIONS
Administration of hypertonic dextrose and amino acid solutions via central venous catheter may be associated with complications which can be prevented or minimized by careful attention to all aspects of the procedure. This includes attention to solution preparation, administration and patient monitoring.
It is essential that carefully prepared protocol, based upon current medical practice, be followed, preferably by an experienced team.
The package insert of the protein (nitrogen) source should be consulted for dosage and all precautionary information.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentration, and acid base balance during prolonged parenteral therapy or whenever the conditions of the patient warrants such evaluation.
Care should be taken to avoid circulatory overload, particularly in patients with cardiac insufficiency.
Caution must be exercised in the administration of these injections to patients receiving corticosteroids or corticotropin.
These injections should be used with caution in patients with overt or subclinical diabetes mellitus.
Drug product contains no more than 25 µg//L of aluminum.
Carcinogenesis and Mutagenesis and Impairment of Fertility
Studies with 50% and 70% Dextrose Injection, USP have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Dextrose Injections, USP. It is also not known whether Dextrose Injections, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dextrose Injections, USP should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Caution should be exercised when 50% and 70% Dextrose Injection, USP is administered to a nursing woman
Pediatric Use
Dextrose is safe and effective for the stated indications in pediatric patients (see INDICATIONS AND USAGE). As reported in the literature, the dosage selection and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia. Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants. Because of their hypertonicity, 50% and 70% Dextrose Injections must be diluted prior to administration.
ADVERSE REACTIONS
Too rapid infusion of a hypertonic dextrose solution may result in diuresis, hyperglycemia, glycosuria, and hyperosmolar coma. Continual clinical monitoring of the patient is necessary in order to identify and initiate measures for these clinical conditions.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
DOSAGE AND ADMINISTRATION
Following suitable admixture of prescribed drugs, the dosage is usually dependent upon age, weight and clinical condition of the patient as well as laboratory determinations. See directions accompanying drugs.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Do not administer unless solution is clear and seal is intact.
Use of a final filter is recommended during administration of all parenteral solutions where possible.
50% and 70% Dextrose Injection, USP in the Pharmacy Bulk Package is intended for use in the preparation of sterile, intravenous admixtures. Additives may be incompatible with the fluid withdrawn from this container. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. When compounding admixtures, use aseptic technique. Mix thoroughly. Do not store any unused portion of the 50% and 70% Dextrose Injection, USP.
DIRECTIONS FOR USE OF VIAFLEX PLASTIC PHARMACY BULK PACKAGE CONTAINER
To Open
Tear overpouch at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired.
For compounding only, not for direct infusion.
Preparation for Admixing
1. The Pharmacy Bulk Package is to be used only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area).
2. Suspend container from eyelet support.
3. Remove plastic protector from outlet port at bottom of container.
4. Attach solution transfer set. Refer to complete directions accompanying set.
Note: The closure shall be penetrated only one time with a suitable sterile transfer device or dispensing set which allows measured dispensing of the contents.
5. The VIAFLEX plastic container should not be written on directly since ink migration has not been investigated. Affix accompanying label for date and time of entry notation.
6. Once container closure has been penetrated, withdrawal of contents should be completed without delay. After initial entry, maintain contents at room temperature (25°C/77°F) and dispense within 4 hours.
HOW SUPPLIED
See Table 1.
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended the product be stored at room temperature (25°C/77°F).
071938462
Baxter Healthcare Corporation
Clintec Nutrition Division
Deerfield, IL 60015 USA
Distributed in Canada by
Baxter Corporation
Toronto, Ontario, Canada
BAXTER, VIAPLEX, and PL 146 are trademarks of Baxter International, Inc.
07-19-38-462 Revised May 2003
| Dextrose (Dextrose monohydrate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Dextrose (Dextrose monohydrate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 02/2008Baxter Healthcare Corporation