BENEFIT BOO BOO ZAP MEDICATED ACNE TREATMENT
-
salicylic acid cream
Benefit Cosmetics, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
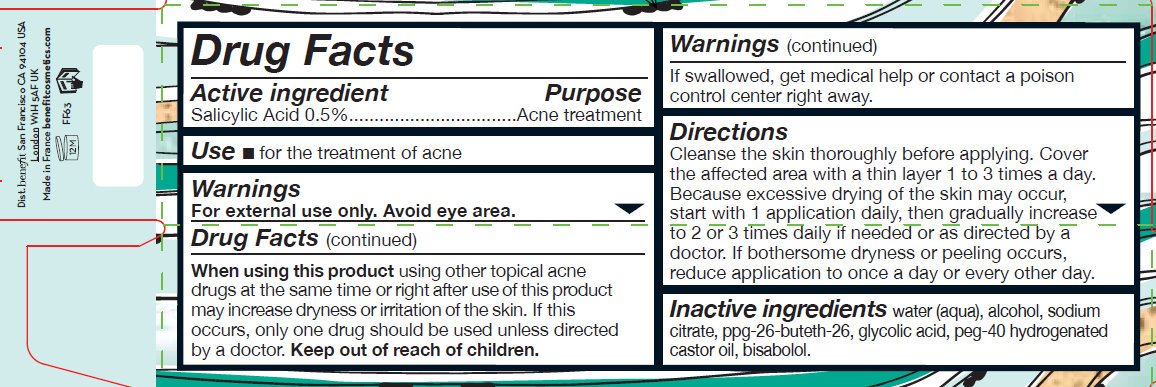
Active Ingredient
Salicylic Acid 0.5%
Use
- for the treatment of acne
Warnings
For external use only. Avoid eye area.
When using this product using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one drug should be used unless directed by a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away.
Directions
Cleanse the skin thoroughly before applying. Cover the affected area with a thin layer 1 to 3 times a day. Because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
water (aqua), alcohol, sodium citrate, ppg-26-buteth-26, glycolic acid, peg-40 hydrogenated castor oil, bisabolol.
Boo boo zap! reduces, clears & helps prevent blemishes from occurring.
Dist. BENEFIT San Francisco CA 94104 USA
London W1H 5AF UK
Made in France benefitcosmetics.com
Benefit BOO BOO ZAP Medicated Acne Treatment 7.4ml (66472-034-01)





BENEFIT BOO BOO ZAP MEDICATED ACNE TREATMENT
salicylic acid cream |
|
|
|
|
|
|
|
|
|
|
Revised: 12/2011 Benefit Cosmetics, LLC





