CHILDRENS MUCINEX CHEST CONGESTION
-
guaifenesin liquid
Reckitt Benckiser LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if the child has
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with asthma
Directions
- do not take more than 6 doses in any 24-hour period
- measure only with dosing cup provided
- do not use dosing cup with other products
- mL = milliliter
| Age | Dose |
|---|---|
| children 6 years to under 12 years | 5 mL – 10 mL every 4 hours |
| children 4 years to under 6 years | 2.5 mL – 5 mL every 4 hours |
| children under 4 years | do not use |
Other information
- each 5 mL contains: sodium 3 mg
- tamper evident: do not use if printed neckband on bottle cap is broken or missing
- store between 20-25°C (68-77°F)
- do not refrigerate
- dosing cup provided
Inactive ingredients
acesulfame potassium, anhydrous citric acid, FD&C blue #1, FD&C red #40, flavor, glycerin, maltitol, propylene glycol, purified water, saccharin sodium, sodium benzoate, sorbitol, sucralose, xanthan gum
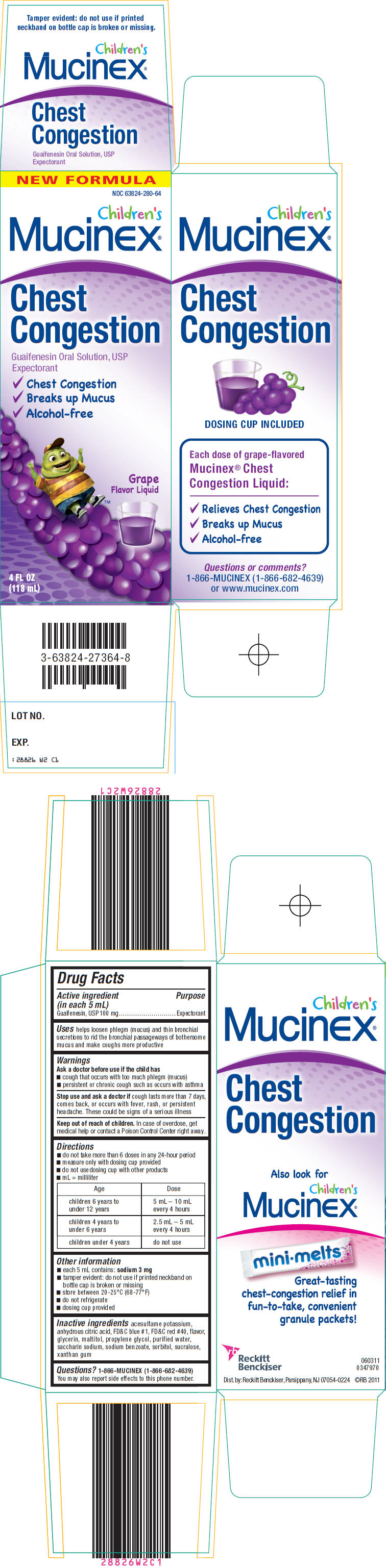
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
NEW FORMULA
NDC 63824-280-64
Children's
Mucinex®
Chest
Congestion
Guaifenesin Oral Solution, USP
Expectorant
- ✓
- Chest Congestion
- ✓
- Breaks up Mucus
- ✓
- Alcohol-free
Grape
Flavor Liquid
4 FL OZ
(118 mL)
| CHILDRENS MUCINEX CHEST CONGESTION
guaifenesin liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part341 | 07/01/2011 | |
| Labeler - Reckitt Benckiser LLC (094405024) |
Revised: 12/2011 Reckitt Benckiser LLC