BRANCHAMIN
-
leucine,
isoleucine and
valine injection, solution
Baxter Healthcare Corporation
----------
DESCRIPTION
4% BranchAmin® (Branched Chain Amino Acid) Injection is a sterile, nonpyrogenic solution of the essential amino acids isoleucine, leucine, and valine provided in a Pharmacy Bulk Package. A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion.
The Viaflex® plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146® Plastic). Exposure to temperatures above 25°C/77ºF during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
Each 100 mL of 4% BranchAmin® (Branched Chain Amino Acid) Injection contains:
| Amino Acids | 4g | |
| Total Nitrogen | 443 mg | |
| pH | 6.0 (5.0 to 7.0) | |
| Osmolarity (calc.) | 316 mOsmol/L | |
CLINICAL PHARMACOLOGY
4% BranchAmin® (Branched Chain Amino Acid) Injection, when appropriately admixed with a complete amino acid injection, such as Travasol® (Amino Acid) Injection, with or without a concentrated calorie source, provides total parenteral nutrition for the severely compromised patient. A common effect of severe stress conditions is depletion of branched chain amino acids. Enriching the standard amino acid solution in TPN with additional branched chain amino acid results in their repletion.
See package insert of the complete amino acid solution for additional information such as Contraindications, Warnings and Precautions pertinent to that drug and general total parenteral nutrition therapy.
INDICATIONS AND USAGE
4% BranchAmin® (Branched Chain Amino Acid) Injection, when appropriately admixed with a complete amino acid injection such as Travasol® Injection, is indicated as an adjunct in offsetting of nitrogen loss or in the treatment of negative nitrogen balance in patients where (1) the alimentary tract cannot or should not he used; (2) gastrointestinal absorption of protein is impaired; or (3) nitrogen homeostasis is substantially impaired as with severe trauma or sepsis. Dosage, route of administration and concomitant infusion of nonprotein calories are dependent on various factors such as nutritional and metabolic status of the patient, anticipated duration of parenteral nutritional support and vein tolerance. See Dosage and Administration for additional information.
Central Vein Administration:
Central venous infusion should be considered when amino acid solutions are to be admixed with hypertonic dextrose to promote protein synthesis such as for hypercatabolic or depleted patients or those requiring long-term parenteral nutrition.
Peripheral Vein Administration:
For patients in whom the central venous route is not indicated, amino acid solutions mixed with low dextrose concentrations may be infused by peripheral vein when supplemented with or without fat emulsion.
Enrichment of the TPN solution with the branched chain amino acid additive is recommended until the patient exhibits a marked reduction in the clinical signs and symptoms of metabolic stress, usually 7 to 14 days.
CONTRAINDICATIONS
4% BranchAmin® (Branched Chain Amino Acid) Injection should not be administered to patients with inborn errors of metabolism, especially those involving branched chain amino acids such as maple syrup urine disease, isovaleric acidemia, etc.
WARNINGS
This injection is for compounding only, not for direct infusion.
Supplementation of branched chain amino acids should occur only with concomitant administration of solutions having complete amino acid profiles and energy substrates designed to meet minimal protein and calorie requirements.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 µg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Hyperammonemia has been reported to occur in infants receiving various amino acid supplementation regimens. Blood ammonia monitoring may be prudent in 4% BranchAmin® infusions.
PRECAUTIONS
General
The concentration of 4% BranchAmin® (Branched Chain Amino Acid) Injection is close to the solubility limits of the components. If exposed to cold, crystals may form. Redissolve any formed crystals by warming to 50ºC. Note: Protect closure from contamination. Do not admix until solution is clear and free of visible particulates.
Drug product contains no more than 25 µg/L of aluminum.
Carcinogenesis and Mutagenesis and Impairment of Fertility
Studies with 4% Branchamin® (Branched Chain Amino Acid) Injection have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with 4% BranchAmin® (Branched Chain Amino Acid) Injection. It is also not known whether 4% BranchAmin® (Branched Chain Amino Acid) Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 4% BranchAmin® (Branched Chain Amino Acid) Injection should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when 4% BranchAmin® (Branched Chain Amino Acid) Injection is administered to a nursing mother.
Pediatric Use
The safety and effectiveness of 4% BranchAmin® (Branched Chain Amino Acid) Injection in pediatric patients have not been established by adequate and well-controlled trials.
Geriatric Use
Clinical studies of 4% BranchAmin® (Branched Chain Amino Acid) Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from other younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or drug therapy.
DOSAGE AND ADMINISTRATION
Clinical studies have shown that stressed patients can safely utilize up to 1.4 g/kg/day of a 50% branched chain amino acid supplemented solution. However, the total daily dose of the branched chain amino acid supplemented solutions depends upon the judgement of the physician based upon the patient's metabolic requirement, branched chain amino acid deficits and clinical response.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions where possible.
Do not administer unless solution is clear and seal is intact.
For dosage and administration of concomitantly administered solutions, reference should be made to the specific product information pertinent to that drug.
Care should be exercised to insure the maintenance of proper levels of serum potassium. Quantities of 60 to 180 mEq of potassium per day have been used with adequate clinical effect. All serum electrolytes should be monitored frequently and electrolyte requirements individualized.
Usual administration of 4% BranchAmin® (Branched Chain Amino Acid) Injection is as a supplement to parenteral nutrition solutions to achieve an amino acid solution which is approximately 50% w/w branched chain amino acid. (One method for achieving this ratio is the admixture of two volumes of 4% BranchAmin® (Branched Chain Amino Acid) Injection at 4 g/100 mL concentration with one volume of an amino acid solution of 8 to 10 g/100 mL concentration.) The supplemental amino acid mixture is administered in combination with energy substrates to provide at least 35 kcal/kg ideal body weight as nonprotein calories.
The treatment regimen should be discontinued when the patient exhibits a marked reduction in the clinical manifestations of metabolic stress, usually 7 to 14 days.
4% BranchAmin® (Branched Chain Amino Acid) Injection in the Pharmacy Bulk Package is intended for use in the preparation of sterile, intravenous admixtures. Additives may be incompatible with the fluid withdrawn from this container. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. When compounding admixtures, use aseptic technique. Mix thoroughly. Do not store any unused portion of 4% BranchAmin® (Branched Chain Amino Acid) Injection.
Directions for Use of Viaflex® plastic Pharmacy Bulk Package container
To Open
Tear overpouch down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired.
For compounding only, not for direct infusion.
Preparation for Admixing
- The Pharmacy Bulk Package is to be used only in a suitable work area such as a laminar flow hood (or an equivalent clean air eompounding area).
- Suspend container from eyelet support.
- Remove plastic protector from outlet port at bottom of container.
- Attach solution transfer set. Refer to complete directions accompanying set. Note: The closure shall be penetrated only one time with a suitable sterile transfer device or dispensing set which allows measured dispensing of the contents.
- Viaflex® containers should not be written on directly since ink migration has not been investigated. Affix accompanying label for date and time of entry.
- Once container closure has been penetrated, withdrawal of contents should be completed without delay. After initial entry, maintain contents at room temperature (25ºC/77ºF) and dispense within 4 hours.
HOW SUPPLIED
4% BranchAmin® (Branched Chain Amino Acid) Injection is available in a Viaflex® plastic Pharmacy Bulk Package container as shown below.
2B6153 500 mL NDC 0338-0477-03
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from excessive cold or freezing. It is recommended the product be stored at room temperature (25ºC/77ºF).
PRINCIPAL DISPLAY PANEL - PACKAGING LABELING

Container Label
LOT
EXP
2B6153
NDC 0338-0477-03
500 mL
4% BranchAmin®
(Branched Chain Amino Acid)
Pharmacy Bulk Package
Not for Direct Infusion
Rx Only
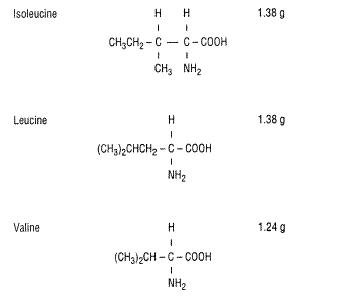
EACH 100 mL CONTAINS ISOLEUCINE 1.38 g LEUCINE 1.38 g VALINE 1.24 g
pH 6.0 (5.0 TO 7.0)
OSMOLARITY 316 mOsmol/L (CALC)
STERILE NONPYROGENIC
CONTAINS NO MORE THAN 25 μg/L OF ALUMINUM
ADDITIVES MAY BE INCOMPATIBLE WITH THE FLUID
WITHDRAWN FROM THIS CONTAINER CONSULT WITH
PHARMACIST IF AVAILABLE WHEN COMPOUNDING
ADMIXTURES USE ASEPTIC TECHNIQUE
MIX THOROUGHLY DO NOT STORE
DOSAGE ADMIX FOR INTRAVENOUS ADMINISTRATION
AS DIRECTED BY A PHYSICIAN SEE ACCOMPANYING
DIRECTIONS FOR USE ONCE CONTAINER CLOSURE HAS
BEEN PENETRATED WITHDRAWAL OF CONTENTS SHOULD
BE COMPLETED WITHOUT DELAY AFFIX ACCOMPANYING LABEL FOR DATE AND TIME OF ENTRY DISPENSE CONTENTS WITHIN 4 HOURS AFTER INITIAL ENTRY
IF EXPOSED TO COLD CRYSTALS MAY FORM REDISSOLVE BY WARMING TO 50ºC
CAUTIONS SQUEEZE AND INSPECT INNER BAG WHICH MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS ARE FOUND DO NOT USE UNLESS SOLUTION IS CLEAR AND SEAL IS INTACT
STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM TEMPERATURE (25ºC/77ºF) UNTIL READY TO USE AVOID EXCESSIVE HEAT PROTECT FROM EXCESSIVE COLD AND FREEZING SEE INSERT
Viaflex® CONTAINER
PL 146 ® PLASTIC
Baxter Logo
BAXTER HEALTHCARE CORPORATION
CLINTEC NUTRITION DIVISION
DEERFIELD IL 60015 USA MADE IN USA
Carton Label
18 - 500 ML
2B6153
VIAFLEX ® CONTAINER
4% BRANCHAMIN® (BRANCHED CHAIN
AMINO ACID) INJECTION
PROTECT FROM
EXCESSIVE COLD AND FREEZING
EXP
XXXXX
SECONDARY BAR CODE
(17) YYMM00 (10) XXXXX
LOT
XXXXX
PRIMARY BAR CODE
(01) 50303380477031
| BRANCHAMIN
leucine, isoleucine and valine injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA018684 | 09/28/1984 | 03/02/2010 |
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Baxter Healthcare Corporation | 059140764 | MANUFACTURE | |
Revised: 12/2011 Baxter Healthcare Corporation