DEXAMETHASONE - dexamethasone injection, solution
Phoenix Pharmaceutical, Inc.
----------
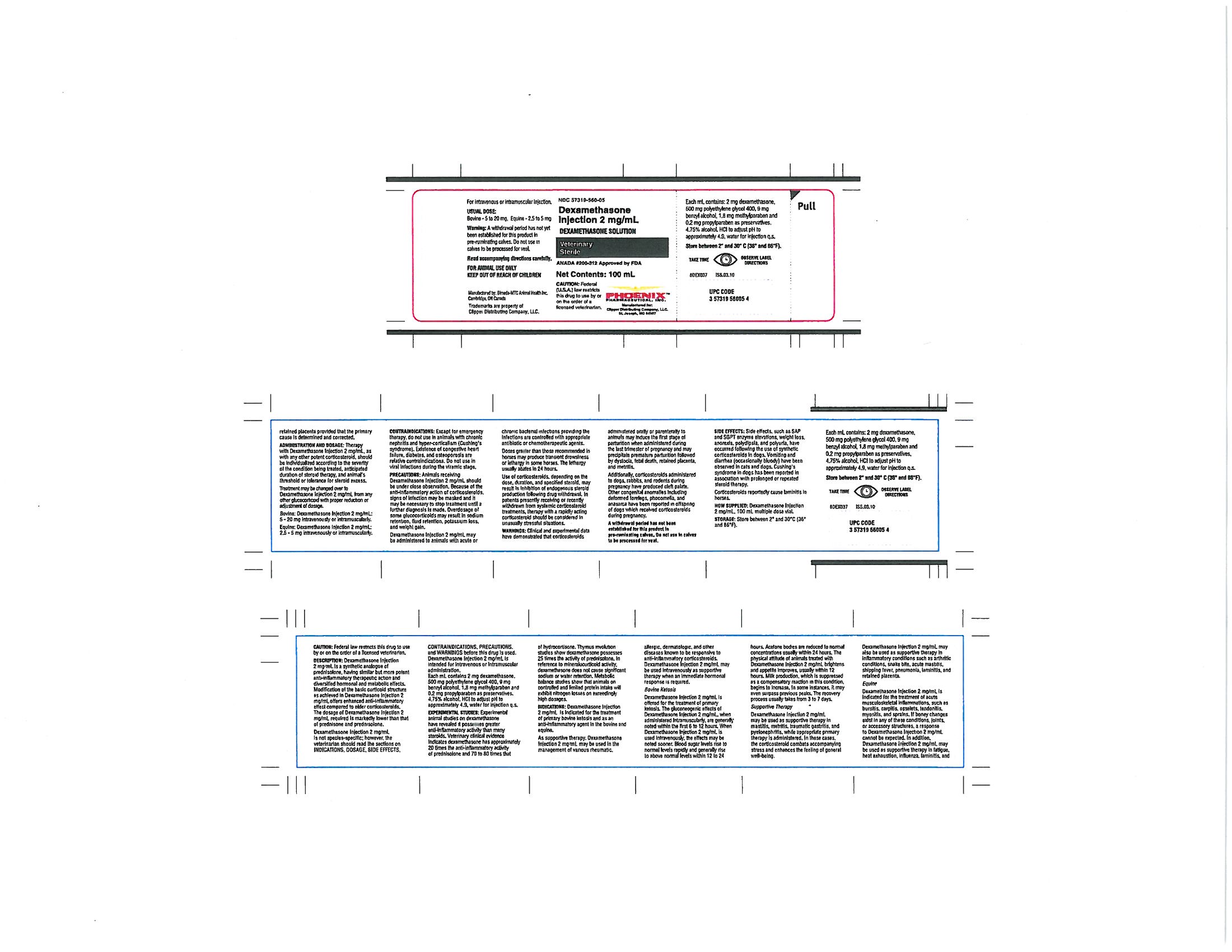
DESCRIPTION:
Dexamethasone Injection 2 mg/mL is a synthetic analogue of prednisolone, having similar but more potent anti-inflammatory therapeutic action and diversified hormonal and metabolic effects. Modification of the basic corticoid structure as achieved in Dexamethasone Injection 2 mg/mL offers enhanced anti-inflammatory effect compared to older corticosteroids. The dosage of Dexamethasone Injection 2 mg/mL required is markedly lower than that of prednisone and prednisolone.
Dexamethasone Injection 2 mg/mL is not species-specific; however, the veterinarian should read the sections on INDICATIONS, DOSAGE, SIDE EFFECTS, CONTRAINDICATIONS, PRECAUTIONS and WARNINGS before this drug is used. Dexamethasone Injection 2 mg/mL is intended for intraveneous or intramuscular administration.
Each mL contains 2 mg dexamethasone, 500mg polyethylene glycol 400, 9 mg benzyl alcohol, 1.8 mg methylparaben and 0.2 mg propylparaben as preservatives, 4.57% alcohol, HCl to adjust pH to approximately 4.9, water for injection q.s.
EXPERIMENTAL STUDIES:
Experimental animal studies on dexamethasone have revealed it possesses greater anti-inflammatory activity than many steroids. Veterinary clinical evidence indicates dexamethasone has approximately 20 times the anti-inflammatory activity of prednisolone and 70 to 80 times that of hydrocortisone. Thymus involution studies show dexamethasone possesses 25 times the activity of prednisolone. In reference to mineralocorticoid activity, dexamethasone does not cause significant sodium or water retention. Metabolic balance studies show that animals on controlled and limited protein intake will exhibit nitrogen losses on exceedingly high dosages.
INDICATIONS:
Dexamethasone Injection 2 mg/mL is indicated for the treatment of primary bovine ketosis and as an anti-inflammatory agent in the bovine and equine.
As supportive therapy, Dexamethasone Injection 2 mg/mL may be used in the management of various rheumatic, allergic, dermatologic, and other diseases known to be responsive to anti-inflammatory corticosteroids. Dexamethasone Injection 2 mg/mL may be used intravenously as supportive therapy when an immediate hormonal response is required.
Bovine Ketosis
Dexamethasone Injection 2 mg/mL is offered for the treatment of primary ketosis. The gluconeogenic effects of Dexamethasone Injection 2 mg/mL, when administered intramuscularly, are generally noted within the first 6 to 12 hours. When Dexamethasone Injection 2 mg/mL is used intraveneously, the effects may be noted sooner. Blood sugar levels rise to normal levels rapidly and generally rise to above normal levels within 12 to 24 hours. Acetone bodies are reduced to normal concentrations usually within 24 hours. The physical attitude of animals treated with Dexamethasone Injection 2 mg/mL brightens and appetite improves, usually within 12 hours. Milk production, which is suppressed as a compensatory reaction in this condition, begins to increase. In some instances, it may even surpass previous peaks. The recovery process usually takes from 3 to 7 days.
Supportive Therapy
Dexamethasone Injection 2 mg/mL may be used as supportive therapy in mastitis, metritis, traumatic gastritis, and pyelonephritis, while appropriate primary therapy is administered. In these cases, the corticosteroid combats accompanying stress and enhances the feeling of general well-being.
Dexamethasone Injection 2 mg/mL may also be used as supportive therapy in inflammatory conditions such as arthritic conditions, snake bite, acute mastitis, shipping fever, pneumonia, laminitis and retained placenta.
Equine
Dexamethasone Injection 2 mg/mL is indicated for the treatment of acute musculoskeletal inflammations, such as bursitis, carpitis, osselets, tendonitis, myositis, and sprains. If boney changes exist in any of these conditions, joints, or accessory structures, a response to Dexamethasone Injection 2 mg/mL cannot be expected. In addition, Dexamethasone Injection 2 mg/mL may be used as supportive therapy in fatigue, heat exhaustion, influenza, laminitis, and retained placenta provided that primary cause is determined and corrected.
ADMINISTRATION AND DOSAGE:
Therapy with Dexamethasone Injection 2 mg/mL, as with any other potent corticosteroid, should be individualized according to the severity of the condition being treated, anticipated duration of steroid therapy, and animal's threshold or tolerance for steroid excess.
Treatment may be changed over to Dexamethasone Injection 2 mg/mL from any other glucocorticoid with proper reduction or adjustment of dosage.
Bovine: Dexamethasone Injection 2 mg/mL; 5 - 20 mg intraveneously or intramuscularly.
Equine: Dexamethasone Injection 2 mg/mL; 5 - 20 mg intraveneously or intramuscularly.
CONTRAINDICATIONS: Except for emergency therapy, do not use in animals with chronic nephritis and hyper-corticalism (Cushing's syndrome). Existence of congestive heart failure, diabetes, and osteoporosis are relative contraindications. Do not use in viral infections during the viremic stage.
PRECAUTIONS: Animals receiving Dexamethasone Injection 2 mg/mL should be under close observation. Because of the anti-inflammatory action of corticosteroids, signs of infection may be masked and it may be necessary to stop treatment until a further diagnosis is made. Overdosage of some glucocorticoids may result in sodium retention, fluid retention, potassium loss, and weight gain.
Dexamethasone Injection 2 mg/mL may be administered to animals with acute or chronic bacterial infections providing the infections are controlled with appropriate antibiotic or chemotherapeutic agents.
Doses greater than those recommended in horses may produce transient drowsiness or lethargy in some horses. The lethargy usually abates in 24 hours.
Use of corticosteroids, depending on the dose, duration, and specified steroid, may result in inhibition or endogenous steroid production following drug withdrawal. in patients presently receiving or recently withdrawn from systemic corticosteroid treatments, therapy with a rapidly acting corticosteroid should be considered in unusually stressful situations.
WARNINGS: Clinical and experimental data have demonstrated that corticosteroids administered orally or parenterally to animals may induce the first stage of parturition when administered during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis.
Additionally, corticosteroids administered to dogs, rabbits, and rodents during pregnancy have produced cleft palate. Other congenital anomalies including deformed forelegs, phocomelia, and anasarca have been reported in offspring of dogs which received corticosteroids during pregnancy.
A withdrawal period has not been established for this product in pre-ruminating calves. Do not use in calves to be processed for veal.
SIDE EFFECTS: Side effects, such as SAP and SGPT enzyme elevations, weight loss, anorexia, polydipsia, and polyuria, have occurred following the use of synthetic corticosterois in dogs. Vomiting and diarrhea (occasionally bloody) have been observed in cats and dogs. Cushing's syndrome in dogs has been reported in association with prolonged or repeated steroid therapy.
Corticosteroids reportedly cause laminitis in horses.
| DEXAMETHASONE
dexamethasone injection, solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Phoenix Pharmaceutical, Inc. (150711039) |
| Registrant - Bimeda, Inc. Division of Cross Vetpharm Group (043653216) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bimeda-MTC Animal Health | 256232216 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Chimie | 291538267 | api manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia and Upjohn | 829076566 | api manufacture | |