anzemet (dolasetron mesylate) tablet, film coated
[sanofi-aventis. U.S. LLC]
DESCRIPTION
ANZEMET (dolasetron mesylate) is an antinauseant and antiemetic agent. Chemically, dolasetron mesylate is (2α,6α,8α,9aβ)-octahydro-3-oxo-2,6 methano-2H-quinolizin-8-yl-1H-indole-3-carboxylate monomethanesulfonate, monohydrate. It is a highly specific and selective serotonin subtype 3 (5-HT3) receptor antagonist both in vitro and in vivo. Dolasetron mesylate has the following structural formula:
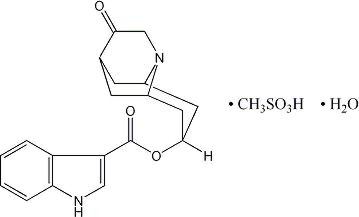
The empirical formula is is C19H20N2O3• CH3SO3H • H2O, with a molecular weight of 438.50. Approximately 74% of dolasetron mesylate monohydrate is dolasetron base.
Dolasetron mesylate monohydrate is a white to off-white powder that is freely soluble in water and propylene glycol, slightly soluble in ethanol, and slightly soluble in normal saline.
Each ANZEMET Tablet for oral administration contains dolasetron mesylate (as the monohydrate) and also contains the inactive ingredients: carnauba wax, croscarmellose sodium, hypromellose, lactose, magnesium stearate, polyethylene glycol, polysorbate 80, pregelatinized starch, synthetic red iron oxide, titanium dioxide, and white wax. The tablets are printed with black ink, which contains lecithin, pharmaceutical glaze, propylene glycol, and synthetic black iron oxide.
CLINICAL PHARMACOLOGY
Dolasetron mesylate and its active metabolite, hydrodolasetron (MDL 74,156), are selective serotonin 5-HT3receptor antagonists not shown to have activity at other known serotonin receptors and with low affinity for dopamine receptors. The serotonin 5-HT3receptors are located on the nerve terminals of the vagus in the periphery and centrally in the chemoreceptor trigger zone of the area postrema. It is thought that chemotherapeutic agents produce nausea and vomiting by releasing serotonin from the enterochromaffin cells of the small intestine, and that the released serotonin then activates 5-HT3receptors located on vagal efferents to initiate the vomiting reflex.
Acute, usually reversible, ECG changes (PR and QTcprolongation; QRS widening), caused by dolasetron mesylate, have been observed in healthy volunteers and in controlled clinical trials. The active metabolites of dolasetron may block sodium channels, a property unrelated to its ability to block 5-HT3receptors. QTcprolongation is primarily due to QRS widening. Dolasetron appears to prolong both depolarization and, to a lesser extent, repolarization time. The magnitude and frequency of the ECG changes increased with dose (related to peak plasma concentrations of hydrodolasetron but not the parent compound). These ECG interval prolongations usually returned to baseline within 6 to 8 hours, but in some patients were present at 24 hour follow up. Dolasetron mesylate administration has little or no effect on blood pressure.
In healthy volunteers (N=64), dolasetron mesylate in single intravenous doses up to 5 mg/kg produced no effect on pupil size or meaningful changes in EEG tracings. Results from neuropsychiatric tests revealed that dolasetron mesylate did not alter mood or concentration. Multiple daily doses of dolasetron have had no effect on colonic transit in humans. Dolasetron has no effect on plasma prolactin concentrations.
Pharmacokinetics in Humans
Oral dolasetron is well absorbed, although parent drug is rarely detected in plasma due to rapid and complete metabolism to the most clinically relevant species, hydrodolasetron.
The reduction of dolasetron to hydrodolasetron is mediated by a ubiquitous enzyme, carbonyl reductase. Cytochrome P-450 (CYP)IID6 is primarily responsible for the subsequent hydroxylation of hydrodolasetron and both CYPIIIA and flavin monooxygenase are responsible for the N-oxidation of hydrodolasetron.
Hydrodolasetron is excreted in the urine unchanged (61.0% of administered oral dose). Other urinary metabolites include hydroxylated glucuronides and N-oxide.
Hydrodolasetron appears rapidly in plasma, with a maximum concentration occurring approximately 1 hour after dosing, and is eliminated with a mean half-life of 8.1 hours (%CV=18%) and an apparent clearance of 13.4 mL/min/kg (%CV=29%) in 30 adults. The apparent absolute bioavailability of oral dolasetron, determined by the major active metabolite hydrodolasetron, is approximately 75%. Orally administered dolasetron intravenous solution and tablets are bioequivalent. Food does not affect the bioavailability of dolasetron taken by mouth.
Hydrodolasetron is eliminated by multiple routes, including renal excretion and, after metabolism, mainly, glucuronidation and hydroxylation. Two thirds of the administered dose is recovered in the urine and one third in the feces. Hydrodolasetron is widely distributed in the body with a mean apparent volume of distribution of 5.8 L/kg (%CV=25%, N=24) in adults.
Sixty-nine to 77% of hydrodolasetron is bound to plasma protein. In a study with14C labeled dolasetron, the distribution of radioactivity to blood cells was not extensive. Approximately 50% of hydrodolasetron is bound to α1-acid glycoprotein. The pharmacokinetics of hydrodolasetron are linear and similar in men and women.
The pharmacokinetics of hydrodolasetron, in special and targeted patient populations following oral administration of dolasetron, are summarized in Table 1. The pharmacokinetics of hydrodolasetron are similar in adult (young and elderly) healthy volunteers and in adult cancer patients receiving chemotherapeutic agents. The apparent clearance following oral administration of hydrodolasetron is approximately 1.6- to 3.4-fold higher in children and adolescents than in adults. The clearance following oral administration of hydrodolasetron is not affected by age in adult cancer patients. The apparent oral clearance of hydrodolasetron decreases 42% with severe hepatic impairment and 44% with severe renal impairment. No dose adjustment is necessary for elderly patients (see PRECAUTIONS, Geriatric Use) or for patients with hepatic or renal impairment.
The pharmacokinetics of ANZEMET Tablets have not been studied in the pediatric population. However, the following pharmacokinetic data are available on intravenous ANZEMET Injection administered orally to children.
Thirty-two pediatric cancer patients ages 3 to 11 years (N=19) and 12 to 17 years (N=13), received 0.6, 1.2, or 1.8 mg/kg ANZEMET Injection diluted with either apple or apple-grape juice and administered orally. In this study, the mean apparent clearances of hydrodolasetron were 3 times greater in the younger pediatric group and 1.8 times greater in the older pediatric group than those observed in healthy adult volunteers. Across this spectrum of pediatric patients, maximum plasma concentrations were 0.6 to 0.7 times those observed in healthy adults receiving similar doses.
For 12 pediatric patients, ages 2 to 12 years receiving 1.2 mg/kg ANZEMET Injection diluted in apple or apple-grape juice and administered orally, the mean apparent clearance was 34% greater and half-life was 21% shorter than in healthy adults receiving the same dose.
| Age (years) |
Dose | CLapp
(mL/min/kg) | t1/2
(h) | Cmax
(ng/mL) |
||
|---|---|---|---|---|---|---|
| CLapp: apparent clearance t1/2: terminal elimination half-life ( ): coefficient of variation in % | ||||||
| Young Healthy Volunteers (N=30) | 19–45 | 200 mg | 13.4 (29%) | 8.1 (18%) | 556 (28%) | |
| Elderly Healthy Volunteers (N=15) | 65–75 | 2.4 mg/kg | 9.5 (36%) | 7.2 (32%) | 662 (28%) | |
| Cancer Patients | ||||||
| Adults (N=61)† | 24–84 | 25–200 mg | 12.9 (49%) | 7.9 (43%) | --‡ | |
| Adolescents (N=13) | 12–17 | 0.6–1.8 mg/kg | 26.5 (67%) | 6.4 (30%) | 374§(32%) | |
| Children (N=19) | 3–11 | 0.6–1.8 mg/kg | 44.2 (49%) | 5.5 (39%) | 217¶(67%) | |
| Pediatric Surgery Patients (N=11) | 2–12 | 1.2 mg/kg | 20.8 (49%) | 5.9 (24%) | 159 (32%) | |
| Patients with Severe Renal Impairment (N=12 (Creatinine clearance ≤10 mL/min) | 28–74 | 200 mg | 7.2 (48%) | 10.7 (29%) | 701 (21%) | |
| Patients with Severe Hepatic Impairment (N=3) | 42–52 | 150 mg | 8.8 (57%) | 11.0 (36%) | 410 (12%) | |
Clinical Studies
Prevention of Cancer Chemotherapy-Induced Nausea and Vomiting
Oral ANZEMET at a dose of 100 mg prevents nausea and vomiting associated with moderately emetogenic cancer therapy as shown by 24 hour efficacy data from two double-blind studies. Efficacy was based on complete response (ie, no vomiting, no rescue medication).
The first randomized, double-blind trial compared single oral ANZEMET doses of 25, 50, 100 and 200 mg in 60 men and 259 women cancer patients receiving cyclophosphamide and/or doxorubicin. There was no statistically significant difference in complete response between the 100 mg and 200 mg dose. Results are summarized in Table 2.
| Response Over 24 Hours | ANZEMET Tablets | ||||
|---|---|---|---|---|---|
| 25 mg (N=78) | 50 mg (N=83) | 100 mg*
(N=80) | 200 mg (N=78) | p-value for Linear Trend |
|
| Complete Response† | 24 (31%) | 34 (41%) | 49 (61%) | 46 (59%) | P<.0001 |
| Nausea Score‡ | 49 | 10 | 11 | 7 | P=.0006 |
Another trial also compared single oral ANZEMET doses of 25, 50, 100, and 200 mg in 307 patients receiving moderately emetogenic chemotherapy. In this study, the 100 mg ANZEMET dose gave a 73% complete response rate.
Prevention of Postoperative Nausea and Vomiting
ANZEMET Tablets at a dose of 100 mg administered orally 1-2 hours before surgery and before general balanced anesthesia (short-acting barbiturate, nitrous oxide, narcotic analgesic, and skeletal muscle relaxant) was significantly more effective than placebo in preventing postoperative nausea and vomiting. Efficacy was based on complete response rates (0 emetic episodes and no rescue medication over 24 hours). No increased efficacy was seen with higher doses.
One trial compared single ANZEMET Tablet doses of 25, 50, 100, and 200 mg with placebo in 789 women undergoing gynecological surgery. In this study the 100 mg dose produced a complete response rate statistically superior to placebo. The study results are summarized in Table 3.
| Response Over 24 Hours | ANZEMET Tablets | ||||
|---|---|---|---|---|---|
| 25 mg (N=159) | 50 mg (N=166) | 100 mg*
(N=154) | 200 mg (N=154) | Placebo (N=156) |
|
| Complete Response† | 71 (45%) | 95 (57%)‡ | 78 (51%)‡ | 73 (47%)‡ | 55 (35%) |
| Nausea Score§ | 5‡ | 4‡ | 5‡ | 6‡ | 15 |
Another trial also compared single oral ANZEMET doses of 25, 50, 100, and 200 mg with placebo in 373 women undergoing gynecological surgery. In this study, the 100 mg ANZEMET dose gave a 54% complete response rate as compared to the 29% rate of placebo.
INDICATIONS AND USAGE
ANZEMET Tablets are indicated for:
- 1)
- the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy, including initial and repeat courses;
- 2)
- the prevention of postoperative nausea and vomiting.
CONTRAINDICATIONS
ANZEMET Tablets are contraindicated in patients known to have hypersensitivity to the drug.
WARNINGS
ANZEMET can cause ECG interval changes (PR, QTc, JT prolongation and QRS widening). These changes are related in magnitude and frequency to blood levels of the active metabolite. These changes are self-limiting with declining blood levels. Some patients have interval prolongations for 24 hours or longer. Interval prolongation could lead to cardiovascular consequences, including heart block or cardiac arrhythmias. These have rarely been reported.
A cardiac conduction abnormality observed on an intra-operative cardiac rhythm monitor (interpreted as complete heart block) was reported in a 61-year-old woman who received 200 mg ANZEMET for the prevention of postoperative nausea and vomiting. This patient was also taking verapamil. A similar event also interpreted as complete heart block was reported in one patient receiving placebo.
A 66-year-old man with Stage IV non-Hodgkins lymphoma died suddenly 6 hours after receiving 1.8 mg/kg (119 mg) intravenous ANZEMET Injection. This patient had other potential risk factors including substantial exposure to doxorubicin and concomitant cyclophosphamide.
Pediatric Use
Dolasetron should be administered with caution in pediatric patients who have or may develop prolongation of cardiac conduction intervals, particularly QTc. Rare cases of sustained supraventricular and ventricular arrhythmias, cardiac arrest leading to death, and myocardial infarction have been reported in children and adolescents(See Precautions, General, and Adverse Reactions – Postmarketing Experience).
PRECAUTIONS
General
Dolasetron should be administered with caution in patients who have or may develop prolongation of cardiac conduction intervals, particularly QTc. These include patients with hypokalemia or hypomagnesemia, patients taking diuretics with potential for inducing electrolyte abnormalities, patients with congenital QT syndrome, patients taking anti-arrhythmic drugs or other drugs which lead to QT prolongation, and cumulative high dose anthracycline therapy.
Cross hypersensitivity reactions have been reported in patients who received other selective 5-HT3receptor antagonists. These reactions have not been seen with dolasetron mesylate.
Drug Interactions
The potential for clinically significant drug-drug interactions posed by dolasetron and hydrodolasetron appears to be low for drugs commonly used in chemotherapy or surgery, because hydrodolasetron is eliminated by multiple routes. See PRECAUTIONS, Generalfor information about potential interaction with other drugs that prolong the QTcinterval. Blood levels of hydrodolasetron increased 24% when dolasetron was coadministered with cimetidine (nonselective inhibitor of cytochrome P-450) for 7 days, and decreased 28% with coadministration of rifampin (potent inducer of cytochrome P-450) for 7 days.
ANZEMET has been safely coadministered with drugs used in chemotherapy and surgery. As with other agents which prolong ECG intervals, caution should be exercised in patients taking drugs which prolong ECG intervals, particularly QTc.
In patients taking furosemide, nifedipine, diltiazem, ACE inhibitors, verapamil, glyburide, propranolol, and various chemotherapy agents, no effect was shown on the clearance of hydrodolasetron. Clearance of hydrodolasetron decreased by about 27% when dolasetron mesylate was administered intravenously concomitantly with atenolol. ANZEMET did not influence anesthesia recovery time in patients. Dolasetron mesylate did not inhibit the antitumor activity of four chemotherapeutic agents (cisplatin, 5-fluorouracil, doxorubicin, cyclophosphamide) in four murine models.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
In a 24-month carcinogenicity study, there was a statistically significant (P<0.001) increase in the incidence of combined hepatocellular adenomas and carcinomas in male mice treated with 150 mg/kg/day and above. In this study, mice (CD-1) were treated orally with dolasetron mesylate 75, 150, or 300 mg/kg/day (225, 450 or 900 mg/m2/day). For a 50 kg person of average height (1.46 m2body surface area), these doses represent 3, 6, and 12 times the recommended clinical dose (74 mg/m2) on a body surface area basis. No increase in liver tumors was observed at a dose of 75 mg/kg/day in male mice and at doses up to 300 mg/kg/day in female mice.
In a 24-month rat (Sprague-Dawley) carcinogenicity study, oral dolasetron mesylate was not tumorigenic at doses up to 150 mg/kg/day (900 mg/m2/day, 12 times the recommended human dose based on body surface area) in male rats and 300 mg/kg/day (1800 mg/m2/day, 24 times the recommended human dose based on body surface area) in female rats.
Dolasetron mesylate was not genotoxic in the Ames test, the rat lymphocyte chromosomal aberration test, the Chinese hamster ovary (CHO) cell (HGPRT) forward mutation test, the rat hepatocyte unscheduled DNA synthesis (UDS) test or the mouse micronucleus test.
Dolasetron mesylate was found to have no effect on fertility and reproductive performance at oral doses up to 100 mg/kg/day (600 mg/m2/day, 8 times the recommended human dose based on body surface area) in female rats and up to 400 mg/kg/day (2400 mg/m2/day, 32 times the recommended human dose based on body surface area) in male rats.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Teratology studies have not revealed evidence of impaired fertility or harm to the fetus due to dolasetron mesylate. These studies have been performed in pregnant rats at oral doses up to 100 mg/kg/day (8 times the recommended human dose based on body surface area) and pregnant rabbits at oral doses up to 100 mg/kg/day (16 times the recommended human dose based on body surface area). There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether dolasetron mesylate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ANZEMET Tablets are administered to a nursing woman.
Pediatric Use
Dolasetron should be administered with caution in pediatric patients who have or may develop prolongation of cardiac conduction intervals, particularly QTc. Rare cases of sustained supraventricular and ventricular arrhythmias, cardiac arrest leading to death, and myocardial infarction have been reported in children and adolescents(See Warningsand Adverse Reactions – Postmarketing Experience).
Geriatric Use
Prevention of cancer chemotherapy-induced nausea and vomiting (CINV)
In controlled clinical trials in the prevention of chemotherapy-induced nausea and vomiting, 301 (29%) of 1026 patients were 65 years of age or older. Of the 301 geriatric patients in the trial, 282 received oral ANZEMET Tablets. No overall differences in safety or effectiveness were observed between geriatric and younger patients, and other reported clinical experience has not identified differences in responses between geriatric and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Prevention and treatment of post-operative nausea and vomiting (PONV)
Controlled clinical studies in the prevention and treatment of post-operative nausea and vomiting did not include sufficient numbers of patients aged 65 years or older – only 5 (0.4%) geriatric patients (all 5 received intravenous ANZEMET Injection) out of 1167 total patients participated in the controlled PONV trials – to determine whether they respond differently from the younger patients. Other reported clinical experiences have not identified differences in responses between geriatric and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
The pharmacokinetics, including clearance of oral ANZEMET Tablets, in elderly and younger patients are similar (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Humans). Dosage adjustment is not needed in patients over the age of 65.
ADVERSE REACTIONS
Chemotherapy Patients
In controlled clinical trials, 943 adult cancer patients received ANZEMET Tablets. These patients were receiving concurrent chemotherapy, predominantly cyclophosphamide and doxorubicin regimens. The following adverse events were reported in ≥2% of patients receiving either ANZEMET 25 mg or ANZEMET 100 mg tablets for prevention of cancer chemotherapy induced nausea and vomiting in controlled clinical trials (Table 4).
| Event | ANZEMET | |
|---|---|---|
| 25 mg (N=235) | 100 mg (N=227) |
|
| Headache | 42 (17.9%) | 52 (22.9%) |
| Fatigue | 6 (2.6%) | 13 (5.7%) |
| Diarrhea | 5 (2.1%) | 12 (5.3%) |
| Bradycardia | 12 (5.1%) | 9 (4.0%) |
| Dizziness | 3 (1.3%) | 7 (3.1%) |
| Pain | 0 | 7(3.1%) |
| Tachycardia | 7 (3.0%) | 6 (2.6%) |
| Dyspepsia | 7 (3.0%) | 5 (2.2%) |
| Chills/Shivering | 3 (1.3%) | 5 (2.2%) |
Postoperative Patients
In controlled clinical trials, 936 adult female patients have received oral ANZEMET for the prevention of postoperative nausea and vomiting. Following is a listing of all adverse events reported in ≥ 2% of patients receiving either placebo or ANZEMET for prevention of postoperative nausea and vomiting in controlled clinical trials (Table 5).
| Event | ANZEMET 100 mg (N=228) | Placebo (N=231) |
|---|---|---|
| Headache | 16 (7.0%) | 11 (4.8%) |
| Hypotension | 12 (5.3%) | 15 (6.5%) |
| Dizziness | 10 (4.4%) | 0 (0.0%) |
| Fever | 8 (3.5%) | 7 (3.0%) |
| Pruritus | 7 (3.1%) | 8 (3.5%) |
| Oliguria | 6 (2.6%) | 3 (1.3%) |
| Hypertension | 5 (2.2%) | 7 (3.0%) |
| Tachycardia | 5 (2.2%) | 2 (0.9%) |
In clinical trials, the following infrequently reported adverse events, assessed by investigators as treatment-related or causality unknown, occurred following oral or intravenous administration of ANZEMET to adult patients receiving concomitant cancer chemotherapy or surgery:
Cardiovascular:Hypotension; rarely–edema, peripheral edema. The following events also occurred rarely and with a similar frequency as placebo and/or active comparator: Mobitz I AV block, chest pain, orthostatic hypotension, myocardial ischemia, syncope, severe bradycardia, and palpitations. See PRECAUTIONSsection for information on potential effects on ECG.
In addition, the following asymptomatic treatment-emergent ECG changes were seen at rates less than or equal to those for active or placebo controls: bradycardia, T wave change, ST-T wave change, sinus arrhythmia, extrasystole (APCs or VPCs), poor R-wave progression, bundle branch block (left and right), nodal arrhythmia, U wave change, atrial flutter/fibrillation.
Furthermore, severe hypotension, bradycardia and syncope have been reported immediately or closely following IV administration.
Dermatologic:Rash, increased sweating.
Gastrointestinal System:Constipation, dyspepsia, abdominal pain, anorexia; rarely–pancreatitis.
Hearing, Taste and Vision:Taste perversion, abnormal vision; rarely–tinnitus, photophobia.
Hematologic:Rarely–hematuria, epistaxis, prothrombin time prolonged, PTT increased, anemia, purpura/hematoma, thrombocytopenia.
Hypersensitivity:Rarely–anaphylactic reaction, facial edema, urticaria.
Liver and Biliary System:Transient increases in AST (SGOT) and/or ALT (SGPT) values have been reported as adverse events in less than 1% of adult patients receiving ANZEMET in clinical trials. The increases did not appear to be related to dose or duration of therapy and were not associated with symptomatic hepatic disease. Similar increases were seen with patients receiving active comparator. Rarely–hyperbilirubinemia, increased GGT.
Metabolic and Nutritional:Rarely–alkaline phosphatase increased.
Musculoskeletal:Rarely–myalgia, arthralgia.
Nervous System:Flushing, vertigo, paresthesia, tremor; rarely–ataxia, twitching.
Psychiatric:Agitation, sleep disorder, depersonalization; rarely–confusion, anxiety, abnormal dreaming.
Respiratory System:Rarely–dyspnea, bronchospasm.
Urinary System:Rarely–dysuria, polyuria, acute renal failure.
Vascular (Extracardiac):Local pain or burning on IV administration; rarely–peripheral ischemia, thrombophlebitis/phlebitis.
Postmarketing Experience
Rare cases of sustatined supraventricular and ventricular arrhythmias, cardiac arrest leading to death, and myocardial infarction have been reported in children and adolescents.
OVERDOSAGE
A 59-year-old man with metastatic melanoma and no known pre-existing cardiac conditions developed severe hypotension and dizziness 40 minutes after receiving a 15 minute intravenous infusion of 1000 mg (13 mg/kg) of dolasetron mesylate. Treatment for the overdose consisted of infusion of 500 mL of a plasma expander, dopamine, and atropine. The patient had normal sinus rhythm and prolongation of PR, QRS and QTcintervals on an ECG recorded 2 hours after the infusion. The patient's blood pressure was normal 3 hours after the event and the ECG intervals returned to baseline on follow-up. The patient was released from the hospital 6 hours after the event.
Following a suspected overdose of ANZEMET Injection, a patient found to have second-degree or higher AV conduction block with ECG should undergo cardiac telemetry monitoring.
There is no known specific antidote for dolasetron mesylate, and patients with suspected overdose should be managed with supportive therapy. Individual doses as large as 5 mg/kg intravenously or 400 mg orally have been safely given to healthy volunteers or cancer patients.
It is not known if dolasetron mesylate is removed by hemodialysis or peritoneal dialysis.
A 7-year-old boy received 6 mg/kg of dolasetron mesylate orally before surgery. No symptoms occurred and no treatment was required.
Single intravenous doses of dolasetron mesylate at 160 mg/kg in male mice and 140 mg/kg in female mice and rats of both sexes (6.3 to 12.6 times the recommended human dose based on body surface area) were lethal. Symptoms of acute toxicity were tremors, depression and convulsions.
DOSAGE AND ADMINISTRATION
The recommended doses of ANZEMET Tablets should not be exceeded.
Prevention of Cancer Chemotherapy-Induced Nausea and Vomiting
Adults
The recommended oral dosage of ANZEMET (dolasetron mesylate) is 100 mg given within one hour before chemotherapy.
Pediatric Patients
The recommended oral dosage in pediatric patients 2 to 16 years of age is 1.8 mg/kg given within one hour before chemotherapy, up to a maximum of 100 mg. Safety and effectiveness in pediatric patients under 2 years of age have not been established.
Use in the Elderly, Renal Failure Patients, or Hepatically Impaired Patients
No dosage adjustment is recommended. (See Pharmacokinetics in Humans.)
Prevention of Postoperative Nausea and Vomiting
Adults
The recommended oral dosage of ANZEMET (dolasetron mesylate) is 100 mg within two hours before surgery.
Pediatric Patients
The recommended oral dosage in pediatric patients 2 to 16 years of age is 1.2 mg/kg given within two hours before surgery, up to a maximum of 100 mg. Safety and effectiveness in pediatric patients under 2 years of age have not been established.
Use in the Elderly, Renal Failure Patients, or Hepatically Impaired Patients
No dosage adjustment is recommended. (See Pharmacokinetics in Humans.)
HOW SUPPLIED
| ANZEMET®
Tablets (dolasetron mesylate) |
|||
|---|---|---|---|
| Strength | Quantity | NDC Number | Description |
| 50 mg | 5 ct Bottle 10 ct Unit Dose Pack | 0088-1202-05 0088-1202-43 | Light pink, film coated, round tablet imprinted with "A" on one side and"50"on the other. |
| 100 mg | 5 ct Bottle 10 ct Unit Dose 5 ct Blister Pack | 0088-1203-05 0088-1203-43 0088-1203-29 | Pink, film coated, elongated oval tablet imprinted with "100" on one side and "ANZEMET" on the other. |
Store at controlled room temperature 20–25 C (68–77 F). Protect from light.
Prescribing Information as of October 2007
Manufactured by: Patheon Pharmaceuticals Inc.
Cincinnati, OH 45237
Manufactured for: sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
| Anzemet (dolasetron mesylate) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Anzemet (dolasetron mesylate) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Revised: 01/2008sanofi-aventis. U.S. LLC