BENZOYL PEROXIDE
-
benzoyl peroxide solution
E. FOUGERA & CO. A division of Nycomed US Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION
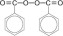
Benzoyl Peroxide Pads 4.5%, Benzoyl Peroxide Pads 6.5% and Benzoyl Peroxide Pads 8.5% are intended for topical administration and contain benzoyl peroxide for use in the treatment of acne vulgaris. Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following chemical structure:
Benzoyl Peroxide Pads 4.5% contain 4.5% solution of benzoyl peroxide on a textured pad. Each gram of medicated solution contains 45 mg benzoyl peroxide, carbomer interpolymer A, edetate disodium, glycerin, purified water, triethanolamine and urea (10%).
Benzoyl Peroxide Pads 6.5% contain 6.5% solution of benzoyl peroxide on a textured pad. Each gram of medicated solution contains 65 mg benzoyl peroxide, carbomer interpolymer A, edetate disodium, glycerin, purified water, triethanolamine and urea (10%).
Benzoyl Peroxide Pads 8.5% contain 8.5% solution of benzoyl peroxide on a textured pad. Each gram of medicated solution contains 85 mg benzoyl peroxide, carbomer interpolymer A, edetate disodium, glycerin, purified water, triethanolamine and urea (10%).
CLINICAL PHARMACOLOGY
The mechanism of action of benzoyl peroxide is not totally understood but its antibacterial activity against Propionibacterium acnes is thought to be a major mode of action. In addition, patients treated with benzoyl peroxide show a reduction in lipids and free fatty acids, and mild desquamation (drying and peeling activity) with simultaneous reduction in comedones and acne lesions.
Little is known about the percutaneous penetration, metabolism, and excretion of benzoyl peroxide, although it has been shown that benzoyl peroxide absorbed by the skin is metabolized to benzoic acid and then excreted as benzoate in the urine. There is no evidence of systemic toxicity caused by benzoyl peroxide in humans.
INDICATIONS AND USAGE
Benzoyl Peroxide Pads are indicated for the topical treatment of acne vulgaris.
CONTRAINDICATIONS
These preparations are contraindicated in patients with a history of hypersensitivity to any of their components.
PRECAUTIONS
General: For external use only. If severe irritation develops, discontinue use and institute appropriate therapy. After reaction clears, treatment may often be resumed with less frequent application. These preparations should not be used in or near the eyes or on mucous membranes.
Information for Patients: Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. Contact with any colored material (including hair and fabric) may result in bleaching or discoloration. If excessive irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Data from several studies employing a strain of mice that is highly susceptible to developing cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of these findings to humans is unknown. Benzoyl peroxide has not been found to be mutagenic (Ames Test) and there are no published data indicating it impairs fertility.
Pregnancy: Teratogenic Effects: Pregnancy Category C: Animal reproduction studies have not been conducted with benzoyl peroxide. It is not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed. There are no available data on the effect of benzoyl peroxide on the later growth, development and functional maturation of the unborn child.
ADVERSE REACTIONS
Allergic contact dermatitis and dryness have been reported with topical benzoyl peroxide therapy.
OVERDOSAGE
If excessive scaling, erythema or edema occurs, the use of this preparation should be discontinued. To hasten resolution of the adverse effects, cool compresses may be used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not allergenicity.
DOSAGE AND ADMINISTRATION
Benzoyl Peroxide Pads: Apply to affected areas once or twice a day, or as directed by your physician. Wet skin and liberally apply to areas to be cleansed, massage gently into skin for 10-20 seconds, avoiding eyes. Lightly rinse and pat dry.
If excessive drying occurs, it may be controlled by rinsing product off sooner and/or using less often.
HOW SUPPLIED
Benzoyl Peroxide Pads 4.5% contain 30 Foil Pouches, each with a single-use medicated pad (6 mL each), NDC 0168-0476-01.
Benzoyl Peroxide Pads 6.5% contain 30 Foil Pouches, each with a single-use medicated pad (6 mL each), NDC 0168-0477-01.
Benzoyl Peroxide Pads 8.5% contain 30 Foil Pouches, each with a single-use medicated pad (6 mL each), NDC 0168-0478-01.
Store at 15°-25° C (59°-77° F). Protect from freezing.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Mfd. for:
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747
Mfd. by: Pegasus Laboratories, Inc.
Pensacola, FL 32514
IL303A
Iss. 09/08
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BENZOYL PEROXIDE PADS 8.5% - 6 mL MEDICATED PAD
NDC 0168-0478-01
6mL
Fougera
Benzoyl Peroxide
Pads 8.5%
In a Urea Vehicle
Not for Ophthalmic, Oral or Vaginal Use
For Topical Use Only
Rx only
Contains: 1 single-use
medicated pad (6 mL)
Single-Use Pad / Discard After Use
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BENZOYL PEROXIDE PADS 8.5% - CARTON OF 30 FOIL POUCHES
NDC 0168-0478-01
30 Foil Pouches (6mL each)
Fougera
Benzoyl Peroxide
Pads 8.5%
In a urea Vehicle
Not for Ophthalmic, Oral or Vaginal Use
For Topical Use Only
Contains: 30 Foil Pouches, each with a
single-use medicated pad (6 mL each)
Single-Use Pad / Discard After Use
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BENZOYL PEROXIDE PADS 6.5% - 6 mL MEDICATED PAD
NDC 0168-0477-01
6mL
Fougera
Benzoyl Peroxide
Pads 6.5%
In a Urea Vehicle
Not for Ophthalmic, Oral or Vaginal Use
For Topical Use Only
Rx only
Contains: 1 single-use
medicated pad (6 mL)
Single-Use Pad / Discard After Use
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BENZOYL PEROXIDE PADS 6.5% - CARTON OF 30 FOIL POUCHES
NDC 0168-0477-01
30 Foil Pouches (6mL each)
Fougera
Benzoyl Peroxide
Pads 6.5%
In a urea Vehicle
Not for Ophthalmic, Oral or Vaginal Use
For Topical Use Only
Contains: 30 Foil Pouches, each with a
single-use medicated pad (6 mL each)
Single-Use Pad / Discard After Use
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BENZOYL PEROXIDE PADS 4.5% - 6 mL MEDICATED PAD
NDC 0168-0476-01
6mL
Fougera
Benzoyl Peroxide
Pads 4.5%
In a Urea Vehicle
Not for Ophthalmic, Oral or Vaginal Use
For Topical Use Only
Rx only
Contains: 1 single-use
medicated pad (6 mL)
Single-Use Pad / Discard After Use
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BENZOYL PEROXIDE PADS 4.5% - CARTON OF 30 FOIL POUCHES
NDC 0168-0476-01
30 Foil Pouches (6mL each)
Fougera
Benzoyl Peroxide
Pads 4.5%
In a urea Vehicle
Not for Ophthalmic, Oral or Vaginal Use
For Topical Use Only
Contains: 30 Foil Pouches, each with a
single-use medicated pad (6 mL each)
Single-Use Pad / Discard After Use
E. FOUGERA & CO.
A division of Nycomed US Inc.
Melville, New York 11747
| BENZOYL PEROXIDE
benzoyl peroxide solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 08/15/2009 | 11/30/2011 | |
| BENZOYL PEROXIDE
benzoyl peroxide solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 08/15/2009 | 11/30/2011 | |
| BENZOYL PEROXIDE
benzoyl peroxide solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 08/15/2009 | 04/30/2011 | |
| Labeler - E. FOUGERA & CO. A division of Nycomed US Inc. (043838424) |
| Registrant - Nycomed US Inc. (043838424) |
Revised: 12/2011 E. FOUGERA & CO. A division of Nycomed US Inc.