KERAFOAM 42
-
urea aerosol, foam
Onset Dermatologics LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION:
KERAFOAM® 42 Emollient Foam is a keratolytic emollient
foam which is a tissue softener for skin and/or nails. Each gram of KERAFOAM 42
Emollient Foam contains 42% urea USP, ceteareth-10 phosphate, cetearyl alcohol
NF, dicetyl phosphate, dl-alpha tocopheryl acetate USP, edetate disodium dihydrate
USP, methylparaben NF, propylene glycol USP, propylparaben NF, purified water USP,
sodium phosphate monobasic USP. Also contains: Propellant HFA-134a (1,1,1,2-
tetrafluoroethane).
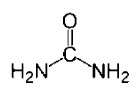
Urea USP is a diamide of carbonic acid with the following
chemical structure:
CLINICAL PHARMACOLOGY
Urea gently lyses/dissolves the intercellular matrix of surface skin cells loosening and allowing a shedding of rough, thickened and scaly hyperkeratotic skin. Urea also moisturizes and softens skin.
INDICATIONS
For softening, smoothing and removing rough scaling hyperkeratotic skin in conditions such as xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
CONTRAINDICATIONS
Known hypersensitivity to any of the listed ingredients. Discontinue use if hypersensitivity is observed.
WARNINGS
FOR EXTERNAL USE ONLY. Avoid contact with eyes, lips or mucous membranes. Keep out of the reach of children. Contents under pressure. Do not puncture or incinerate container. Do not expose to temperatures above 120ºF (49ºC).
Information for patients:
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with KERAFOAM 42 Emollient Foam. It is not known whether KERAFOAM 42 Emollient Foam can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. KERAFOAM 42 Emollient Foam should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether or not this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when KERAFOAM 42 Emollient Foam is administered to a nursing woman.
ADVERSE REACTIONS
Transient stinging, burning, itching or irritation may occur and normally disappear on discontinuing the medication.
DOSAGE AND ADMINISTRATION
Shake Vigorously, Tap Bottom of Can, and Prime Before
Initial Use. Shake Vigorously and Tap Before Each Use.
To Prime: After shaking, gently tap bottom of can onto palm of other hand or a solid
surface at least 3 times. Hold the can upright, direct away from the patient, and
firmly depress the actuator for 1 to 3 seconds or until foam begins to dispense. (If
foam does not dispense within 3 seconds: re-shake can, gently tap bottom of can onto a
solid surface at least 3 times, and depress the actuator again until foam begins to
dispense.)
Before Each Use: Shake vigorously and gently tap bottom of can onto palm of other hand or a
solid surface at least 3 times.
During Use: Holding can upright, dispense KERAFOAM 42 into palm of hand and apply to
affected area twice per day, or as directed by a physician. Rub in until completely
absorbed. Wipe off any excess foam from actuator after use.
HOW SUPPLIED
KERAFOAM 42 Emollient Foam is supplied in a 60 g (NDC# 16781-181-60) and 100g (NDC# 16781-181-96) aluminum cans.
Store at room temperature: 59º - 77ºF (15º - 25ºC). Protect from freezing. Store upright.
Patent Pending
P/N 2614 Rev. 1
Manufactured For:

Onset Therapeutics
Cumberland, RI 02864
(888) 713-8154
www.onsettx.com
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Kerafoam42-Outer Box - 5g
NDC 16781-181-06
Rx Only
Kerafoam® 42
Emollient Foam Urea (42%)
For softening, smoothing, and removing rough scaling hyperkeratotic skin
See prescribing information enclosed
Professional Sample
Enclosed: Six 5g Samples
Available in 60g and 100g Cans

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Kerafoam42-Inner Label - 5g
NDC 16781-181-06
Rx Only
Professional Sample
Not for Sale
Net Weight 5g
Kerafoam® 42
Emollient Foam Urea (42%)
Sample will not dispense entire contents.

| KERAFOAM 42
urea aerosol, foam |
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 04/01/2009 | 12/31/2011 | |
| Labeler - Onset Dermatologics LLC (793223707) |
| Registrant - Onset Dermatologics LLC (964275155) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Onset Dermatologics LLC | 793223707 | Manufacture | |
Revised: 11/2011 Onset Dermatologics LLC