ITCHY EYE
-
ketotifen solution
Major Pharmaceuticals
----------
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
Directions
- Adults and children 3 years of age and older:
Put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day.
- Children under 3 years of age:
Consult a doctor.
Other information
- only for use in the eye
- store at 20-25°C (68-77°F) [see USP Controlled Room Temperature]
Inactive ingredients
benzalkonium chloride 0.01%, glycerin, purified water. May contain hydrochloric acid and/or sodium hydroxide (to adjust pH).
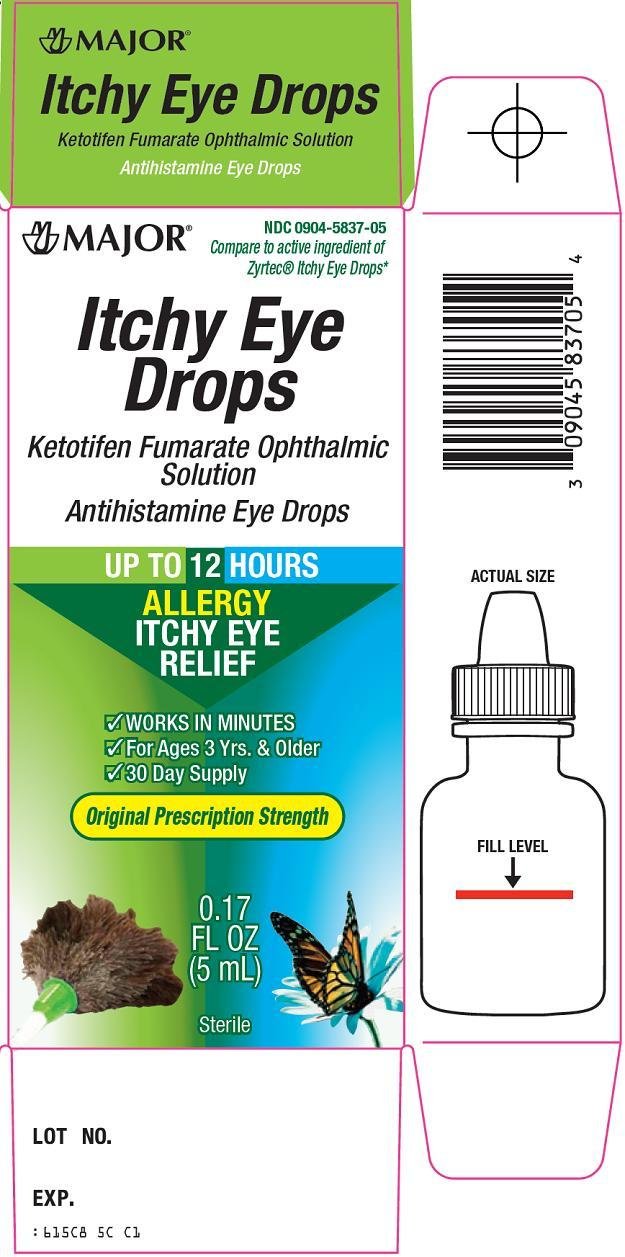
Package/Label Principal Display Panel
Compare to active ingredient of Zyrtec® Itchy Eye Drops
Itchy Eye Drops
Ketotifen Fumarate Ophthalmic Solution
Antihistamine Eye Drops
UP TO 12 HOURS
ALLERGY ITCHY EYE RELIEF
WORKS IN MINUTES
For Ages 3 Yrs. & Older
30 Day Supply
Original Prescription Strength
Sterile
Itchy Eye Drops Carton Image 1

Itchy Eye Drops Carton Image 2
| ITCHY EYE
ketotifen solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA077958 | 11/16/2010 | |
| Labeler - Major Pharmaceuticals (191427277) |
Revised: 11/2011 Major Pharmaceuticals