DALLERGY PSE
-
chlorpheniramine maleate,
pseudoephedrine hydrochloride and
methscopolamine nitrate tablet, extended release
Laser Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DESCRIPTION:
Each Dallergy® PSE Tablet contains:
Chlorpheniramine Maleate ................................................4 mg
Pseudoephedrine Hydrochloride .....................................10 mg
Methscopolamine Nitrate ...............................................1.25 mg
Chlorpheniramine Maleate is an antihistamine having the chemical name 2-pyridinepropanamine, γ-(4 chlorphenyl)-N,N-dimethyl, (Z)-2-butenedioate (1:1), having the following structural formula:
Figure 1: Chlorpheniramine Maleate
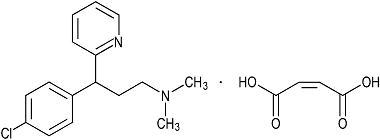
C16H19ClN2·C4H4O4 M.W. 390.86
Pseudoephedrine hydrochloride is a nasal decongestant with the chemical name Benzenemethanol, α-[1-(methylamino)ethyl]-, [S-(R*,R*)-, hydrochloride. Its structure is as follows:
Figure 2: Pseudoephedrine Hydrochloride
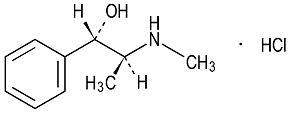
C10H15NO·HCl M.W. 201.69
Methscopolamine is an anticholinergic having the chemical name 3-Oxa-9-azoniatricyclo[3.3.1.02,4] nonane, 7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9,9-dimethyl-, nitrate, [7-(S)-(1α,2β,4β,5α,7β)]-, having the following structural formula:
Figure 3: Methscopolamine Nitrate
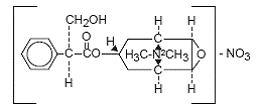
C17H24NO4· NO3 M.W. 380.4
Dallergy® PSE Tablets contain ingredients of the following therapeutic classes: antihistamine, nasal decongestant, and anticholinergic agent.
INACTIVE INGREDIENTS
calcium phosphate, lactose monohydrate, magnesium stearate, methylcellulose, microcrystalline cellulose, povidone, and stearic acid
CLINICAL PHARMACOLOGY:
Chlorpheniramine Maleate is an alkylamine-type antihistamine which acts by competing with histamine for H1 histamine receptor sites, thereby preventing the action of histamine on the cell. Clinically, Chlorpheniramine suppresses the histamine-mediated symptoms of allergic rhinitis, relieving sneezing, rhinorrhea, and itching of the eyes, nose, and throat.
Pseudoephedrine hydrochloride is an orally active sympathomimetic amine and exerts a decongestant action on the nasal mucosa. Pseudoephedrine hydrochloride is recognized as an effective agent for the relief of nasal congestion due to allergic rhinitis. Pseudoephedrine produces peripheral effects similar to those of ephedrine and central effects similar to, but less intense than, amphetamines. It has the potential for excitatory side effects.
Methscopolamine nitrate is a quaternary ammonium derivative of scopolamine, which possesses the peripheral actions of the belladonna alkaloids, but does not exhibit the central actions because of its lack of ability to cross the blood-brain barrier. In this formulation, it is used because of its antisecretory effects on the respiratory system.
INDICATIONS AND USAGE:
For the relief of upper respiratory symptoms associated with allergies and the common cold, such as nasal congestion, sinusitis, sneezing, lacrimation, vasomotor rhinitis, post-nasal drip, and hay fever.
CONTRAINDICATIONS:
Hypersensitivity to any of the ingredients. Also contraindicated in patients with severe hypertension, severe coronary artery disease, patients on MAO inhibitor therapy, patients with narrow angle glaucoma, urinary retention, peptic ulcer, and during an asthmatic attack.
WARNINGS:
Considerable caution should be exercised in patients with hypertension, diabetes mellitus, ischemic heart disease, hyperthyroidism, increased intraocular pressure, and prostatic hypertrophy. The elderly (60 years or older) are more likely to exhibit adverse reactions. Antihistamines may cause excitability, especially in children. At dosages higher than the recommended dose, nervousness, dizziness, or sleeplessness may occur.
PRECAUTIONS:
General:
Caution should be exercised in patients with high blood pressure, heart disease, diabetes, or thyroid disease. The antihistamine in this product may exhibit additive effects with other CNS depressants, including alcohol.
Information for Patients:
Antihistamines may cause drowsiness, and ambulatory patients who operate machinery or motor vehicles should be cautioned accordingly.
Drug Interactions:
MAO inhibitors and beta adrenergic blockers increase the effects of sympathomimetics. Sympathomimetics may reduce the antihypertensive effects of methyldopa, mecamylamine, reserpine and veratrum alkaloids. Concomitant use of antihistamines with alcohol and other CNS depressants may have an additive effect.
Pregnancy:
Pregnancy Category C: It is not known whether Dallergy® PSE Tablets can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dallergy® PSE Tablets should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS:
Adverse reactions include drowsiness, lassitude, nausea, giddiness, dryness of mouth, blurred vision, cardiac palpitations, flushing, increased irritability or excitement (especially in children).
OVERDOSAGE AND TREATMENT OF OVERDOSE:
In all cases of suspected overdose, immediately call your regional poison control center, and/or contact a physician immediately. The stomach should be emptied promptly by lavage or by induction of emesis with Syrup of Ipecac. The installation of activated charcoal into the stomach also should be considered. The treatment of overdose is essentially symptomatic and supportive. If respiratory depression is present, treat promptly with oxygen and/or mechanical support of ventilation. If convulsions or marked CNS excitement occurs, only short-acting benzodiazepine-type drugs should be used.
DOSAGE AND ADMINISTRATION:
Adults and children 12 years of age and older: 1 to 2 tablets every 12 hours.
Children 6 to under 12 years of age: 1 tablet every 12 hours.
Children under 6 years of age: Not recommended
Not to exceed 2 doses in 24 hours.
HOW SUPPLIED
Dallergy® PSE Tablets: Bottles of 100 (NDC 16477-146-01) white, modified capsule shaped tablets debossed with “LAS 146” on one side and plain white on the other side.
| DALLERGY PSE
chlorpheniramine maleate, pseudoephedrine hcl, and methscopolamine nitrate tablet, extended release |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 01/21/2011 | 08/30/2011 | |
| Labeler - Laser Pharmaceuticals, LLC (614417132) |
| Registrant - Laser Pharmaceuticals, LLC (614417132) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sovereign Pharmaceuticals, LLC | 623168267 | MANUFACTURE | |
Revised: 01/2011 Laser Pharmaceuticals, LLC
