HEALTHY ACCENTS MALDROXAL
-
aluminum hydroxide,
magnesium hydroxide and
dimethicone liquid
DZA Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
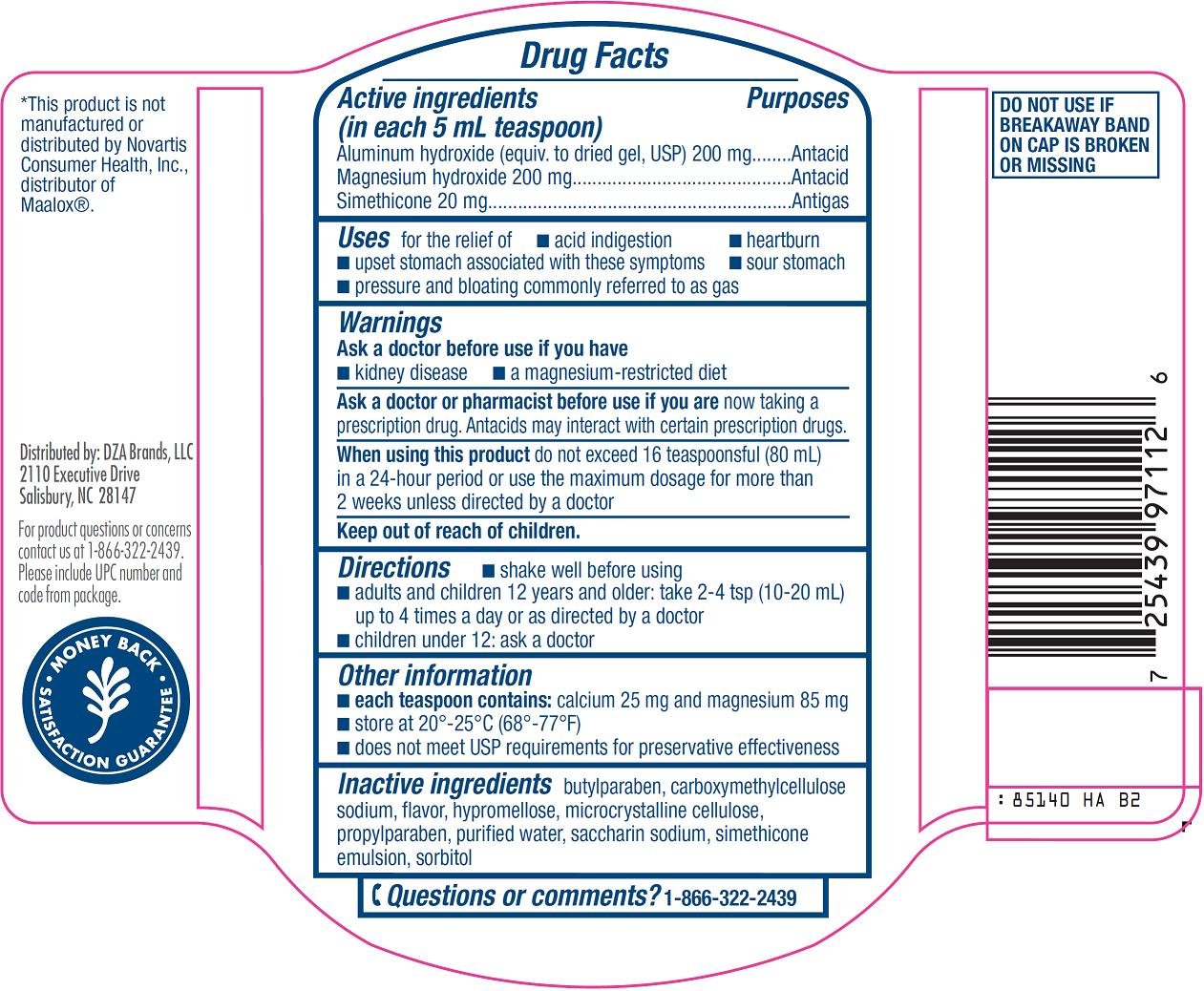
Active ingredient (in each 5 mL teaspoon)
Aluminum hydroxide (equiv. to dried gel, USP) 200 mg
Magnesium hydroxide 200 mg
Simethicone 20 mg
Uses
for the relief of
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
- pressure and bloating commonly referred to as gas
Warnings
Ask a doctor or pharmacist before use if you are
now taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
- shake well before using
- adults and children 12 years and older: take 2-4 tsp (10-20 mL) up to 4 times a day or as directed by a doctor
- children under 12: ask a doctor
Other information
- each teaspoon contains: calcium 25 mg and magnesium 85 mg
- store at 20°-25°C (68°-77°F)
- does not meet USP requirements for preservative effectiveness
Inactive ingredients
butylparaben, carboxymethylcellulose sodium, flavor, hypromellose, microcrystalline cellulose, propylparaben, purified water, saccharin sodium, simethicone emulsion, sorbitol
Principal Display Panel
regular strength
maldroxal®
antacid plus anti-gas
fast relief from:
heartburn
acid indigestion
sour stomach
Compare to Maalox® active ingredients
cooling mint

Maldroxal Front Label
Maldroxal Back Label
| HEALTHY ACCENTS MALDROXAL
aluminum hydroxide, magnesium hydroxide, simethicone liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part332 | 01/29/2008 | |
| Labeler - DZA Brands LLC (090322194) |
Revised: 10/2011 DZA Brands LLC