hydergine (ergoloid mesylates) tablet
hydergine (ergoloid mesylates) liquid
hydergine lc (ergoloid mesylates) capsule, liquid filled
[Novartis Pharmaceuticals Corporation]
T1999-75
Hydergine®
(ergoloid mesylates) Tablets, USP (ORAL)
(ergoloid mesylates) Liquid, USP
Hydergine® LC
(ergoloid mesylates, USP) Liquid Capsules
Rx only
DESCRIPTION
Hydergine® Tablet 1 mg and
Hydergine® LC Liquid Capsule 1 mg
Each contains ergoloid mesylates, USP as follows: dihydroergocornine mesylate 0.333 mg, dihydroergocristine mesylate 0.333 mg, and dihydroergocryptine (dihydro-alpha-ergocryptine and dihydro-beta-ergocryptine in the proportion of 2:1) mesylate 0.333 mg, representing a total of 1 mg.
Inactive Ingredients
Oral Tablets: lactose, povidone, starch, stearic acid, and talc.
Liquid Capsules: ascorbic acid, gelatin, glycerin, methylparaben, polyethylene glycol, propylparaben, propylene glycol, sorbitol, and titanium dioxide.
Hydergine® Liquid 1 mg/mL
Each mL contains ergoloid mesylates, USP as follows: dihydroergocornine mesylate 0.333 mg, dihydroergocristine mesylate 0.333 mg, and dihydroergocryptine (dihydro-alpha-ergocryptine and dihydro-beta-ergocryptine in the proportion of 2:1) mesylate 0.333 mg, representing a total of 1mg; alcohol, 28.5% by volume.
Inactive Ingredients: alcohol, glycerin, propylene glycol, and purified water.
Pharmacokinetic Properties
Pharmacokinetic studies have been performed in normal volunteers with the help of radiolabelled drug as well as employing a specific radioimmunoassay technique. From the urinary excretion quotient of orally and intravenously administered tritium-labelled Hydergine® (ergoloid mesylates) the absorption of ergoloid was calculated to be 25%. Following oral administration, peak levels of 0.5 ngEq/mL/mg were achieved within 1.5-3 hr. Bioavailability studies with the specific radioimmunoassay confirm that ergoloid is rapidly absorbed from the gastrointestinal tract, with mean peak levels of 0.05-0.13 ng/mL/mg (with extremes of 0.03 and 0.18 ng/mL/mg) achieved within 0.6-1.3 hr (with extremes of 0.4 and 2.8 hr). The finding of lower peak levels of ergoloid compared to the total drug-metabolite composite is consistent with a considerable first pass liver metabolism, with less than 50% of the therapeutic moiety reaching the systemic circulation. The elimination of radioactivity, representing ergoloid plus metabolites bearing the radiolabel, was biphasic with half-lives of 4 and 13 hr. The mean half-life of unchanged ergoloid in plasma is about 2.6-5.1 hr; after 3 half-lives ergoloid plasma levels are less than 10% of radioactivity levels, and by 24 hr no ergoloid is detectable.
Bioequivalence studies were performed comparing Hydergine® (ergoloid mesylates) oral tablets (administered orally) with Hydergine® (ergoloid mesylates) sublingual tablets (administered sublingually), Hydergine® (ergoloid mesylates) oral tablets with Hydergine® (ergoloid mesylates) liquid, and Hydergine® (ergoloid mesylates) oral tablets with Hydergine® LC (ergoloid mesylates, USP) liquid capsules. The oral tablet, sublingual tablet, and liquid capsule oral forms were shown to be bioequivalent. Within the bioequivalence limits, the liquid capsule showed a statistically significant (12%) greater bioavailability than the oral tablet. In the study comparing the oral tablet and liquid forms, both forms tested showed an equivalent rate of absorption and an equivalent peak plasma concentration (Cmax).
ACTIONS
There is no specific evidence which clearly establishes the mechanism by which Hydergine® (ergoloid mesylates) preparations produce mental effects, nor is there conclusive evidence that the drug particularly affects cerebral arteriosclerosis or cerebrovascular insufficiency.
INDICATIONS
A proportion of individuals over sixty who manifest signs and symptoms of an idiopathic decline in mental capacity (i.e., cognitive and interpersonal skills, mood, self-care, apparent motivation) can experience some symptomatic relief upon treatment with Hydergine® (ergoloid mesylates) preparations. The identity of the specific trait(s) or condition(s), if any, which would usefully predict a response to Hydergine® (ergoloid mesylates) therapy is not known. It appears, however, that those individuals who do respond come from groups of patients who would be considered clinically to suffer from some ill-defined process related to aging or to have some underlying dementing condition (i.e., primary progressive dementia, Alzheimer’s dementia, senile onset, multi-infarct dementia).
Before prescribing Hydergine® (ergoloid mesylates), the physician should exclude the possibility that the patient’s signs and symptoms arise from a potentially reversible and treatable condition. Particular care should be taken to exclude delirium and dementiform illness secondary to systemic disease, primary neurological disease, or primary disturbance of mood. Hydergine® (ergoloid mesylates) preparations are not indicated in the treatment of acute or chronic psychosis, regardless of etiology (see CONTRAINDICATIONS).
The decision to use Hydergine® (ergoloid mesylates) in the treatment of an individual with a symptomatic decline in mental capacity of unknown etiology should be continually reviewed since the presenting clinical picture may subsequently evolve sufficiently to allow a specific diagnosis and a specific alternative treatment. In addition, continued clinical evaluation is required to determine whether any initial benefit conferred by Hydergine® (ergoloid mesylates) therapy persists with time.
The efficacy of Hydergine® (ergoloid mesylates) was evaluated using a special rating scale known as the SCAG (Sandoz Clinical Assessment-Geriatric). The specific items on this scale on which modest but statistically significant changes were observed at the end of twelve weeks include: mental alertness, confusion, recent memory, orientation, emotional lability, self-care, depression, anxiety/fears, cooperation, sociability, appetite, dizziness, fatigue, bothersome(ness), and an overall impression of clinical status.
CONTRAINDICATIONS
Hydergine® (ergoloid mesylates) preparations are contraindicated in individuals who have previously shown hypersensitivity to the drug. Hydergine® (ergoloid mesylates) preparations are also contraindicated in patients who have psychosis, acute or chronic, regardless of etiology.
PRECAUTIONS
Practitioners are advised that because the target symptoms are of unknown etiology, careful diagnosis should be attempted before prescribing Hydergine® (ergoloid mesylates) preparations.
ADVERSE REACTIONS
Hydergine® (ergoloid mesylates) preparations have not been found to produce serious side effects. Transient nausea and gastric disturbances have been reported. Hydergine® (ergoloid mesylates) preparations do not possess the vasoconstrictor properties of the natural ergot alkaloids.
DOSAGE AND ADMINISTRATION
1 mg three times daily.
Alleviation of symptoms is usually gradual and results may not be observed for 3-4 weeks.
HOW SUPPLIED
Hydergine® (ergoloid mesylates) Tablets, USP (Oral)
1 mg
Round, white, engraved “HYDERGINE 1” on one side, “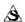 ” other side.
” other side.
NDC 0078-0070-05: bottles of 100
NDC 0078-0070-06: unit-dose packages of 100
NDC 0078-0070-08: bottles of 500
Store and Dispense
Below 77°F (25°C); tight, light-resistant container.
Hydergine® (ergoloid mesylates) Liquid, USP
1 mg/mL
Supplied with an accompanying dropper graduated to deliver 1 mg.
NDC 0078-0100-36: bottles of 100 mL
Store and Dispense
Below 86°F (30°C); tight, amber glass bottle.
Hydergine® LC (ergoloid mesylates, USP) Liquid Capsule
1 mg
Oblong, off-white, branded “HYDERGINE LC 1 mg”
on one side, “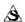 ” other side.
” other side.
NDC 0078-0101-05: bottles of 100
NDC 0078-0101-06: unit-dose packages of 100
NDC 0078-0101-08: bottles of 500
Store and Dispense
Between 59° and 77°F (15° and 25°C); tight, light-resistant container. DO NOT FREEZE.
(Encapsulated by R. P. Scherer, N.A., Clearwater, Florida 33518)
Tablets manufactured by:
Novartis Pharmaceuticals Canada Inc
Dorval (Quebec), Canada H9S 1A9
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
T1999-75
©1999 Novartis
| Hydergine (ergoloid mesylates) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Hydergine (ergoloid mesylates) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Hydergine LC (ergoloid mesylates) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Revised: 05/2006