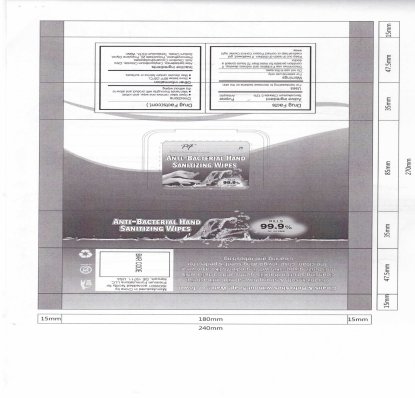
ANTI-BACTERIAL HAND SANITIZING WIPES
-
benzalkonium chloride liquid
American Hygienics Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only.
Do not use in the eyes.
Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children. If swallowed, get medical help or contact Poison Control Center right away.
Open label, remove one wipe, and unfold.
Wet hands thoroughly with product and allow to dry without wiping.
Other information
Store below 95 degree Fahrenheit (35 degree Celsius)
May discolor certain fabrics or surfaces.
Inactive ingredients
Aloe barbadensis, Cetylpyridinium Chloride, Citric Acid, Disodium Cocoamphodiacetate, Phenoxyethanol, Polysorbate 20, Propylene Glycol, Sodium Citrate, Tetrasodium EDTA, Water.
Manufactured in China by
ISO9001 accredited facility for
Premium Formulations LLC,
Newark, DE 19711, USA
Cleans and Refreshes without Soap, Water or Towel
Our thick, soft and strong have a mild antibacterial cleansing solution that kiss germs and bacteria while moisturizing your skin with ingredients like aloe vera. The clean, crisp, invigorating scent is perfect for cleaning and refreshing.
ANTIBACTERIAL HAND WIPES ARE IDEAL FOR:
Home, Office, Car, Purse, Camping, Gym, Boating, Travelling, Anywhere a convenient, antibacterial clean up is required.
| ANTI-BACTERIAL HAND SANITIZING WIPES
benzalkonium chloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part333E | 03/23/2010 | 06/30/2011 |
| Labeler - American Hygienics Corporation (545198454) |
| Registrant - American Hygienics Corporation (545198454) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| American Hygienics Corporation | 545198454 | manufacture | |
Revised: 06/2011 American Hygienics Corporation