totect (dexrazoxane hydrochloride)
[TopoTarget A/S]
Totect™
500 mg*
dexrazoxane for injection
*Each vial of dexrazoxane for injection contains 589 mg dexrazoxane hydrochloride equivalent to 500 mg dexrazoxane
DESCRIPTION
Totect™ (dexrazoxane for injection) is a sterile, pyrogen-free lyophilizate intended for intravenous (IV) administration. Totect™ is provided in a carton consisting of 10 vials of Totect™ and 10 vials of diluent.
Chemically, dexrazoxane is 2,6-piperazinedione,4,4'-(1-methyl-1,2-ethanediyl) bis-,(S)- or (S)-(+)-1,2-bis(3,5-dioxopiperazin-1-yl)propane. The following diagram shows the chemical structure:
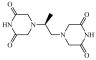
The molecular formula is C11H16N4O4; the molecular weight is 268.3. Dexrazoxane is a white to off-white powder, with a melting point of 194 ± 3 °C. It is soluble in dioxane and 0.1 N HCl, sparingly soluble in water, tetrahydrofuran, citrate buffer at pH 4.0, phosphate buffer at pH 7.0, and borate-potassium chloride sodium hydroxide buffer at pH 9.0. The acid dissociation constants, pKa, are 2.5 (for the tertiary piperazine nitrogen) and 9.7 (for the nitrogen imide). The log P is -2.135.
The finished product is supplied in a sterile form for intravenous infusion only following mixing and diluting.
Each carton contains twenty 50 mL Type I glass vials. Ten vials contain dexrazoxane hydrochloride equivalent to 500 mg dexrazoxane. The other 10 vials contain diluent (0.167M Sodium Lactate Injection, USP). Each vial of dexrazoxane for injection is closed with an aluminum flip-off cap covered with a dark red overcap. Each vial of diluent is closed with an aluminum flip-off cap covered with a white overcap.
When reconstituted as directed, the admixture contains dexrazoxane and the following excipients: hydrochloric acid, sodium lactate, water for injection, sodium hydroxide and lactic acid (see DOSAGE AND ADMINISTRATION). The admixture should be further diluted in 0.9% NaCl prior to administration to patients.
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism by which Totect™ diminishes tissue damage resulting from the extravasation of anthracycline drugs is unknown. Some evidence suggests that dexrazoxane inhibits topoisomerase II reversibly.
Pharmacokinetics/Pharmacodynamics
The pharmacokinetics of dexrazoxane following dosing of patients with anthracycline extravasation have not been studied.
The pharmacokinetics of dexrazoxane have been studied in advanced cancer patients with normal renal and hepatic function. Generally, the pharmacokinetics of dexrazoxane can be adequately described by a two-compartment open model with first-order elimination. Dexrazoxane has been administered as a 15 minute infusion over a dose-range of 60 to 900 mg/m2 with 60 mg/m2 of doxorubicin, and at a fixed dose of 500 mg/m2 with 50 mg/m2 doxorubicin. The disposition kinetics of dexrazoxane are dose-independent, as shown by linear relationship between the area under plasma concentration-time curves and administered doses ranging from 60 to 900 mg/m2. The mean peak plasma concentration of dexrazoxane was 36.5 μg/mL at the end of the 15 minute infusion of a 500 mg/m2 dose of dexrazoxane administered 15 to 30 minutes prior to the 50 mg/m2 doxorubicin dose. The important pharmacokinetic parameters of dexrazoxane are summarized in the following table.
|
a Coefficient of variation |
||||||
|
b Steady-state volume of distribution |
||||||
| Dose | Dose | Number of | Elimination | Plasma | Renal | bVolume of |
| Doxorubicin | Dexrazoxane | Subjects | Half-Life | Clearance | Clearance | Distribution |
| (mg/m2) | (mg/m2) | (h) | (L/h/m2) | (L/h/m2) | (L/m2) | |
| 50 | 500 | 10 | 2.5 (16) | 7.88 (18) | 3.35 (36) | 22.4 (22) |
| 60 | 600 | 5 | 2.1 (29) | 6.25 (31) | — | 22.0 (55) |
Following a rapid distributive phase (~0.2 to 0.3 hours), dexrazoxane reaches post-distributive equilibrium within 2 to 4 hours. The estimated steady-state volume of distribution of dexrazoxane suggests its distribution primarily in the total body water (25 L/m2). The mean systemic clearance and steady-state volume of distribution of dexrazoxane in two Asian female patients at 500 mg/m2 dexrazoxane along with 50 mg/m2 doxorubicin were 15.15 L/h/m2 and 36.27 L/m2, respectively, but their elimination half-life and renal clearance of dexrazoxane were similar to those of the ten Caucasian patients from the same study. Qualitative metabolism studies with dexrazoxane have confirmed the presence of unchanged drug, a diacid-diamide cleavage product, and two monoacid-monoamide ring products in the urine of animals and man. The metabolite levels were not measured in the pharmacokinetic studies.
Urinary excretion plays an important role in the elimination of dexrazoxane. Forty-two percent of the 500 mg/m2 dose of dexrazoxane was excreted in the urine.
Protein Binding: In vitro studies have shown that dexrazoxane is not bound to plasma proteins.
Special Populations:
Pediatric: The pharmacokinetics of dexrazoxane have not been evaluated in pediatric patients.
Gender: There are no clinically relevant differences in the pharmacokinetics of dexrazoxane between males and females.
Renal insufficiency: The pharmacokinetics of dexrazoxane were assessed following a single 15 minute IV infusion of 150 mg/m2 of dexrazoxane in male and female subjects with varying degrees of renal dysfunction as determined by creatinine clearance (CLCR) based on a 24-hour urinary creatinine collection. Dexrazoxane clearance was reduced in subjects with renal dysfunction. Compared with controls, the mean AUC0-inf value was twofold greater in subjects with moderate (CLCR 30-50 mL/min) to severe (CLCR <30 mL/min) renal dysfunction. Modeling demonstrated that equivalent exposure (AUC0-inf) could be achieved if dosing were reduced by 50% in subjects with creatinine clearance values < 40 mL/min compared with control subjects (CLCR >80 mL/min) (see PRECAUTIONS, DOSAGE AND ADMINISTRATION).
Hepatic insufficiency: The pharmacokinetics of dexrazoxane have not been evaluated in patients with hepatic impairment.
Drug Interactions: There were no significant changes in the pharmacokinetics of doxorubicin (50 mg/m2) and its predominant metabolite, doxorubicinol, in the presence of dexrazoxane (500 mg/m2) in a crossover study in cancer patients.
CLINICAL STUDIES
Totect™ was studied in two open-label, single arm, multi-center studies testing whether Totect™ administration could reduce tissue injury following anthracycline extravasation and thereby reduce or avoid surgical intervention.
In the two studies, eligible patients were receiving single-agent anthracycline intravenously (usually as part of combination chemotherapy) and developed extravasation symptoms of pain, burning, swelling, and/or redness near the infusion site. Skin biopsy samples from the suspected area were examined for the presence of anthracycline as determined by the presence of tissue fluorescence; however, therapy was not delayed for this test result.
In both studies, treatment with Totect™ was to begin as soon as possible and no later than 6 hours after extravasation with retreatment 24 and 48 hours later (a total of 3 doses). Totect™ was administered as 1-2 hour IV infusions through a different venous access location. The first and second doses were 1000 mg/m2 and the third dose was 500 mg/m2. No dose modifications were planned except for patients whose body surface area exceeded 2.0 m2, in which case the total daily dose limit on the first and second day was 2000 mg/day and 1000 mg on the third day.
In total, 80 patients were enrolled and 57 were evaluable. Demographics in the two studies were similar. The median age was 57 years, and sixty-five percent of patients were women. The anthracyclines most commonly associated with extravasation were epirubicin (56%) and doxorubicin (41%). Peripheral IV sites of extravasation included the forearm in 63%, the hand in 21%, and the antecubital area in 11%; four patients (5%) received the anthracycline via a central venous access device (CVAD). Most patients presented with swelling (83%), redness (78%), and pain (43%). In study 1, 11% also presented with blisters. The median baseline lesion area was 25 cm2 (range 1-253 cm2).
Evaluable patients had to be receiving single-agent IV anthracycline at the time of extravasation, to have skin biopsies showing fluorescence, and to receive the first Totect™ dose within 6 hours of the extravasation.
In study 1, none of the 19 evaluable patients required surgical intervention and none had serious late sequelae. In study 2, one of the 38 evaluable patients required surgery. One additional non-evaluable patient required surgery for tissue necrosis. Thirteen patients had late sequelae at the event site such as site pain, fibrosis, atrophy, and local sensory disturbance; all were judged as mild except in the one patient who required surgery. None of the 4 patients with CVADs required surgical intervention.
INDICATIONS AND USAGE
Totect™ is indicated for the treatment of extravasation resulting from IV anthracycline chemotherapy.
CONTRAINDICATIONS
None known.
WARNINGS
Pregnancy - Pregnancy Category D - Dexrazoxane was toxic to pregnant rats at doses of 2 mg/kg (1/80 the human dose on a mg/m2 basis) and embryotoxic and teratogenic at 8 mg/kg (about 1/20 the human dose on a mg/m2 basis) when given daily during the period of organogenesis. Teratogenic effects in the rat included imperforate anus, microphthalmia, and anophthalmia. In offspring allowed to develop to maturity, fertility was impaired in the male and female rats treated in utero during organogenesis at 8 mg/kg. In rabbits, doses of 5 mg/kg (about 1/16 the human dose on a mg/m2 basis) daily during the period of organogenesis caused maternal toxicity and doses of 20 mg/kg (1/4 the human dose on a mg/m2 basis) were embryotoxic and teratogenic. Teratogenic effects in the rabbit included several skeletal malformations such as short tail, rib and thoracic malformations, and soft tissue variations including subcutaneous, eye and cardiac hemorrhagic areas, as well as agenesis of the gallbladder and of the intermediate lobe of the lung.
There is no adequate information about the use of Totect™ in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
PRECAUTIONS
General
Totect™ is a cytotoxic drug. When administered to patients receiving anthracycline-containing cytotoxic therapy, additive cytotoxicity may occur. Treatment with Totect™ is associated with leukopenia, neutropenia, and thrombocytopenia. Hematological monitoring should be performed. Reversible elevations of liver enzymes may occur with dexrazoxane.
Patients with Moderate or Severe Renal Insufficiency
Greater exposure to dexrazoxane may occur in patients with compromised renal function. The Totect™ dose should be reduced by 50% in patients with creatinine clearance values <40 mL/min (see DOSAGE AND ADMINISTRATION).
Dimethylsulfoxide (DMSO) should not be used in patients who are receiving dexrazoxane to treat anthracycline-induced extravasation.
Information for Patients
Women of who have the potential to become pregnant should be advised that Totect™ might cause fetal harm.
Laboratory Tests
Blood counts and liver enzymes should be monitored.
Drug Interactions
None known.
Carcinogenesis/Mutagenesis/Impairment of Fertility
No carcinogenicity studies have been done with Totect™ in animals. The carcinogenic potential of dexrazoxane has not been investigated. Nevertheless, a study by the National Cancer Institute has reported that long term dosing with razoxane (the racemic mixture of dexrazoxane, ICRF-187, and its enantiomer ICRF-186) is associated with the development of malignancies in rats and possibly in mice. Dexrazoxane was not mutagenic to bacteria in vitro (Ames assay), but caused significant chromosomal aberrations in mammalian cells in vitro. It also increased the formation of micronucleated polychromatic erythrocytes in mice. Thus, dexrazoxane is mutagenic and clastogenic.
The possible adverse effects of Totect™ on the fertility of humans and experimental animals, male or female, have not been adequately studied. Testicular atrophy was seen with dexrazoxane administration at doses as low as 30 mg/kg weekly for 6 weeks in rats (about 1/5 the human dose on a mg/m2 basis) and as low as 20 mg/kg weekly for 13 weeks in dogs (about half the human dose on a mg/m2 basis).
Pregnancy
Category D. See WARNINGS section.
Labor and Delivery
The effect of dexrazoxane on labor and delivery in humans has not been studied.
Nursing Mothers
It is not known whether dexrazoxane or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from dexrazoxane, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of Totect™ in pediatric patients have not been established.
Geriatric Use
In total, 21% of the patients treated with Totect™ were age 65 years or older, and 9 % were 75 and older. No differences in safety or efficacy were observed between older and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
Overview
Dexrazoxane has been studied previously as a cytotoxic agent. Adverse reactions of nausea/vomiting, diarrhea, stomatitis, bone marrow suppression (neutropenia, thrombocytopenia), altered liver function (increased AST/ALT), and infusion site burning have been observed. These adverse reactions have been reversible.
Discussion of Adverse Reaction Information
Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug may not reflect the rates observed in practice.
In the two clinical studies, Totect™ was administered to patients also receiving chemotherapeutic agents for cancer, and the adverse reaction profile reflects the combination of Totect™, underlying disease, and chemotherapy. The adverse reaction data reflect exposure to Totect™ in 80 patients who received the first dose, 72 patients who received two doses, and 69 patients who received all three doses. Table 1 summarizes adverse reaction occurring with ≥ 5% frequency.
| MedDRA System Organ Class (SOC) and Preferred term | Study 1 and 2 Combined (All causalities) N=80 (%) |
| Total number of patients with at least one event | 68 (85) |
| General disorders and administration site conditions | 46 (58) |
| Pyrexia | 17 (21) |
| Injection site pain | 13 (16) |
| Fatigue | 10 (13) |
| Edema peripheral | 8 (10) |
| Injection site phlebitis | 5 (6) |
| Gastrointestinal disorders | 44 (55) |
| Nausea | 34 (43) |
| Vomiting | 15 (19) |
| Diarrhea | 9 (11) |
| Abdominal pain | 5 (6) |
| Constipation | 5 (6) |
| Infections and infestations | 24 (30) |
| Postoperative infection | 13 (16) |
| Nervous system disorders | 19 (24) |
| Dizziness | 9 (11) |
| Headache | 5 (6) |
| Skin and subcutaneous disorders | 14 (18) |
| Alopecia | 11 (14) |
| Respiratory, thoracic and mediastinal disorders | 13 (16) |
| Dyspnea | 6 (8) |
| Pneumonia | 5 (6) |
| Cough | 4 (5) |
| Vascular disorders | 12 (15) |
| Blood and lymphatic system disorders | 11 (14) |
| Anemia | 5 (6) |
| Psychiatric disorders | 11 (14) |
| Depression | 6 (8) |
| Insomnia | 4 (5) |
| Musculoskeletal and connective tissue disorders | 10 (13) |
| Metabolism and nutrition disorders | 8 (10) |
| Anorexia | 4 (5) |
| Cardiac disorders | 4 (5) |
Neutropenia and febrile neutropenia each occurred in 2.5% of patients.
Table 2 summarizes laboratory adverse reactions from studies 1 and 2 combined.
| CTCAE version 3 Term | CTC grade 3 | CTC grade 4 | CTC grade 2 to 4 |
| N (%) | N (%) | N (%) | |
| Hematologic: | |||
| Decreased hemoglobin | 2 (3) | 0 | 34 (43) |
| Decreased WBC | 20 (25) | 16 (20) | 58 (73) |
| Decreased neutrophils | 17 (22) | 19 (24) | 48 (61) |
| Decreased platelets | 17 (21) | 0 | 21 (26) |
| Hepatic: | |||
| Increased bilirubin | 1 (2) | 0 | 6 (11) |
| Increased AST | 1 (1) | 1 (1) | 21 (28) |
| Increased ALT | 1 (1) | 4 (5) | 17 (22) |
| Increased alkaline phosphatase | 0 | 0 | 3 (4) |
| Increased LDH | 0 | 0 | 1 (5) |
| Metabolic: | |||
| Increased creatinine | 1 (2) | 1 (2) | 8 (14) |
| Decreased sodium | 4 (5) | 1 (1) | 5 (6) |
| Increased calcium total | 1 (2) | 1 (2) | 4 (7) |
OVERDOSAGE
There are no data on overdosage in the two clinical studies. There is no known antidote for dexrazoxane.
DOSAGE AND ADMINISTRATION
Vial contents must be mixed and diluted before use.
Dose:
Totect™ should be given once daily for 3 consecutive days. The first infusion should be initiated as soon as possible and within the first six hours after extravasation.
| The recommended dose is: | maximum recommended dose: | |
| Day one: | 1000 mg/m2 | 2000 mg |
| Day two: | 1000 mg/m2 | 2000 mg |
| Day three: | 500 mg/m2 | 1000 mg |
The Totect™ dose should be reduced by 50% in patients with creatinine clearance values <40 mL/min.
Preparation and administration:
The indicated dose should be administered as an intravenous infusion over 1 to 2 hours in a large caliber vein in an extremity/area other than the one affected by the extravasation. Cooling procedures such as ice packs, if used, should be removed from the area at least 15 minutes before Totect™ administration in order to allow sufficient blood flow to the area of extravasation. Treatment on Day 2 and Day 3 should start at the same hour (+/- 3 hours) as on the first day.
Directions for Mixing and Diluting:
Read this entire section carefully before mixing and diluting.
Aseptic technique should be used during preparation.
Totect™ should not be mixed or administered with any other drug during infusion.
The individual dosage is based on calculation of the body surface area (BSA) up to a maximum dose of 2000 mg (each on Day 1 and 2) and 1000 mg (Day 3), corresponding to a BSA of 2 m2.
Totect™ is provided in a carton containing 10 vials of Totect™ (dexrazoxane for injection) and 10 vials of diluent, to provide 3 days of treatment for 1 patient.
Before infusion, each vial of dexrazoxane (contains 589 mg dexrazoxane hydrochloride equivalent to 500 mg dexrazoxane) must be mixed with 50 mL of diluent. The mixed solution should be further diluted in 1000 mL 0.9% NaCl.
Preparation of the Totect™ mixed and diluted solution.
- Each vial of Totect™ (dexrazoxane for injection) (500 mg) must be mixed with 50 mL of diluent.
- The solution should be used immediately (within 2 hours) after preparation. It contains no antibacterial preservative.
- Inject the mixed volume into the infusion bag with 1000 mL 0.9% NaCl. Totect™ must not be mixed with any other drugs.
- Repeat steps 1 and 2. in order to obtain the required dose, and inject all the required mixed solutions into the same 1000 mL 0.9% NaCl bag.
- Totect™ should be infused over 1 to 2 hours at room temperature and normal light conditions.
The solution of Totect™ is slightly yellow.
Parenteral drug products should be inspected visually for particulate matter prior to administration, whenever solution and container permit. Solutions containing a precipitate should be discarded. Vials are for single use only. Unused solution should be discarded.
The reconstituted product should be used immediately after mixing and diluting as it contains no antibacterial preservative. The product is stable for 4 hours from the time of mixing and diluting when stored below 25°C (77°F).
Instructions for handling and disposal
Caution must be exercised when handling TotectTM , preparing the mixed solution and disposing of the product. Procedures for proper handling of cytotoxic drugs should be adopted, as outlined in the references1-5. Direct contact of TotectTM with the skin or mucous membranes prior to and following reconstitution should be avoided. If contact occurs, wash immediately and thoroughly with water.
HOW SUPPLIED
Totect™ is available in the following strength:
NDC 38423-110-01
500 mg dexrazoxane in a single-use vial is packaged in a carton consisting of 10 vials of dexrazoxane for injection and 10 vials of diluent.
Storage
Store at 25°C (77°F); excursions permitted between 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Protect from light. Keep vials in carton until ready for use.
Distributed by:
Integrated Commercialization Solutions
Brooks, KY 40109
USA
Manufactured by:
Ben Venue Laboratories, Inc.
Bedford, Ohio 44146
USA
Hameln Pharmaceuticals GmbH
31789 Hameln
Germany
Manufactured for:
TopoTarget A/S
Symbion Science Park
Fruebjergvej 3
DK-2 100 Copenhagen
Denmark
Marketed by:
TopoTarget USA Inc.
100 Enterprise Drive
Rockaway, New Jersey 07866
USA
Rx Only
REFERENCES:
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- NIH [2002]. 1999 recommendations for the safe handling of cytotoxic drugs. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, NIH Publication No.92-2621.
- American Society of Health-System Pharmacists. (2006) ASHP Guidelines on Handling Hazardous Drugs.
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society.
Totect™ is a trademark of TopoTarget A/S, Copenhagen, Denmark.
US Patent No. 6,727,253 B2
October 2007
TOT-PIL/v02
| Totect (dexrazoxane hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 12/2007TopoTarget A/S