THERACYS
-
bacillus calmette-guerin substrain connaught live antigen injection, powder, lyophilized, for solution
Sanofi Pasteur Inc.
----------
WARNING
TheraCys®, BCG Live (Intravesical) contains live, attenuated mycobacteria. Because of the potential risk for transmission, it should be prepared, handled and disposed of as a biohazard material. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.)
BCG infections have been reported in health care workers, primarily from exposures resulting from accidental needle sticks or skin lacerations during the preparation of BCG for administration. Nosocomial infections have been reported in immunosuppressed patients receiving parenteral drugs that were prepared in areas in which BCG was prepared. BCG is capable of dissemination when administered by the intravesical route and serious infections, including fatal infections, have been reported in patients receiving Intravesical BCG. (See WARNINGS, PRECAUTIONS and ADVERSE REACTIONS.)
DESCRIPTION
TheraCys, BCG Live (Intravesical) is a freeze-dried preparation made from the Connaught strain of Bacillus Calmette and Guérin, which is an attenuated strain of Mycobacterium bovis.
The BCG organisms in the product are grown on media containing potatoes, glycerine, asparagine, citric acid, potassium phosphate, magnesium sulfate, ferric ammonium citrate, calcium chloride, copper sulfate and zinc sulfate. Monosodium glutamate is added to the BCG organisms prior to freeze-drying.
Each vial of TheraCys contains 81 mg of freeze-dried BCG. The freeze-dried BCG does not contain any preservative.
One dose of TheraCys consists of one 81mg vial of freeze-dried BCG reconstituted and diluted in 50 mL sterile, preservative-free saline.
The BCG organisms from the vial are viable. In vitro potency is determined by an assay of live BCG organisms based on the number of colonies observed when grown on solid medium. The reconstituted product contains 10.5 ± 8.7 × 108 colony-forming units (CFU) per vial.
CLINICAL PHARMACOLOGY
When administered intravesically as a cancer therapy, BCG promotes a local acute inflammatory and sub-acute granulomatous reaction with macrophage and lymphocyte infiltration in the urothelium and lamina propria of the urinary bladder. (1) (2) The exact mechanism of action is unknown, but the anti-tumor effect appears to be T-lymphocyte-dependent. (2) (3)
Clinical Studies
In a multicenter randomized clinical trial, TheraCys was compared to doxorubicin hydrochloride (Adriamycin®) in patients with carcinoma in situ (CIS) of the urinary bladder, recurrent Ta/T1 papillary tumors of the urinary bladder, or both. (4) Patients were stratified by the presence or absence of CIS and analyzed separately. All papillary tumors were completely resected prior to study entry. The study endpoints were disease-free survival and 2-year disease-free survival. TheraCys was administered intravesically weekly for 6 weeks, with an additional single instillation at 3, 6, 12, 18 and 24 months following the initiation of treatment (total of 11 instillations over 2 years). The initial treatment with doxorubicin was given within 3 days of TUR, followed by 4 weekly treatments and then by 11 monthly treatments (total of 16 instillations over 1 year). Cytology and cystoscopy were obtained every 3 months for 2 years. A total of 285 patients were randomized: 142 to treatment with doxorubicin (69 CIS and 73 non-CIS) and 143 to treatment with TheraCys (70 CIS and 73 non-CIS). An intent-to-treat analysis was performed.
For patients with CIS, the complete response rate (ie, negative biopsies and urine cytology) within 6 months of the initiation of treatment was 33% with doxorubicin and 71% with TheraCys (p < 0.001, Fisher's Exact Test). The probability of being disease-free at 2 years was 23% with doxorubicin and 51% with TheraCys (p < 0.001, Z Test). The median disease-free survival was 4.9 months for doxorubicin and 30 months for TheraCys (p < 0.001, Log Rank Test).
For patients with Ta/T1 papillary tumors only, the 2-year disease-free survival was 29% with doxorubicin and 50% with TheraCys (p = 0.008, Z Test). The median disease-free survival was 10.5 months with doxorubicin and 22.5 months with TheraCys (p = 0.001, Log Rank Test).
The results are summarized in Table 1.
| Carcinoma in situ | Ta/T1 Papillary Tumors | |||
|---|---|---|---|---|
| Doxorubicin N = 69 | TheraCys N = 70 | Doxorubicin N = 73 | TheraCys N = 73 |
|
|
||||
| Complete Response | 23 (33%) | 50 (71%) | - | - |
| Median Disease- free Survival* | 4.9 Months | 30 Months | 10.5 Months | 22.5 Months |
| 2-Year Disease- free Survival* | 23% | 51% | 29% | 50% |
| 95% Confidence Interval | (15%, 35%) | (41%, 65%) | (20%, 41%) | (39%, 63%) |
INDICATIONS AND USAGE
TheraCys is indicated for intravesical use in the treatment and prophylaxis of carcinoma in situ (CIS) of the urinary bladder and for the prophylaxis of primary or recurrent stage Ta and/or T1 papillary tumors following transurethral resection (TUR). TheraCys is not recommended for stage TaG1 papillary tumors, unless they are judged to be at high risk of tumor recurrence.
TheraCys is not indicated as an immunizing agent for the prevention of tuberculosis.
CONTRAINDICATIONS
Because of the risk of disseminated BCG infection, TheraCys should not be used in immunosuppressed patients or persons with congenital or acquired immune deficiencies, whether due to concurrent disease (eg, AIDS, leukemia, lymphoma), cancer therapy (eg, cytotoxic drugs, radiation), or immunosuppressive therapy (eg, corticosteroids). (See PRECAUTIONS, Drug Interactions.)
TheraCys is contraindicated for patients with current symptoms or a previous history of systemic BCG reaction. (See Warnings.)
Treatment should be postponed until resolution of a concurrent febrile illness, urinary tract infection, or macroscopic hematuria.
Seven to 14 days should elapse before TheraCys is administered following biopsy, TUR, or traumatic catheterization.
TheraCys should not be administered to persons with active tuberculosis. Active tuberculosis should be ruled out before starting treatment with TheraCys. A test for detecting Mycobacterium tuberculosis infection should be performed if PPD (purified protein derivative of tuberculin) status is unknown. A positive Mantoux test, by itself, is not a contraindication to using TheraCys but an assessment must be made regarding whether the patient has signs, symptoms and/or a chest x-ray consistent with active or latent tuberculosis that requires treatment with antimycobacterial drugs. (5)
WARNINGS
Systemic BCG Reaction
A systemic BCG reaction is a systemic granulomatous illness, which may occur subsequent to exposure to BCG. Because it is usually difficult to isolate BCG organisms from affected organs, it is often unclear to what extent such a reaction is caused by an infectious process versus an inflammatory hypersensitivity reaction, hence the term "systemic BCG reaction". Based on past clinical experience with intravesical BCG, "systemic BCG reaction" may be defined as the presence of any of the following signs, if no other etiologies for such signs are detectable: fever ≥39.5°C (≥103.1°F) for ≥12 hours; fever ≥38.5°C (≥101.3°F) for ≥48 hours; pneumonitis; hepatitis; other organ dysfunction outside of the genitourinary tract with granulomatous inflammation on biopsy; or the classical signs of sepsis, including circulatory collapse, acute respiratory distress and disseminated intravascular coagulation. (6) If TheraCys is administered within one week of either biopsy, TUR or traumatic bladder catheterization (associated with hematuria), a systemic BCG reaction is much more likely to occur. Death has been reported with the use of TheraCys in association with systemic BCG reaction in post-marketing experience.
Patients should be monitored for the presence of symptoms and signs of toxicity after each intravesical treatment. If a patient develops persistent fever or experiences an acute febrile illness consistent with BCG infection, BCG instillations should be permanently discontinued, the patient immediately evaluated and treated for BCG infection and an infectious diseases consultation sought. (See PRECAUTIONS.)
Additional Warnings
TheraCys is not a vaccine for the prevention of cancer.
TheraCys is an infectious agent. Physicians using this product should be familiar with the literature on the prevention and treatment of BCG-related complications and should be prepared in such emergencies to contact an infectious disease specialist with experience in treating the infectious complications of intravesical BCG. The treatment of the infectious complications of BCG requires long-term, multiple-drug antimycobacterial therapy. Special culture media are required for mycobacteria and physicians administering intravesical BCG should have these media readily available.
The use of TheraCys may cause tuberculin sensitivity. Since this is a valuable aid in the diagnosis of tuberculosis, it may be advisable to determine the tuberculin reactivity by testing before treatment.
Intravesical instillations of BCG should be postponed during treatment with antibiotics, since antimicrobial therapy may interfere with the effectiveness of TheraCys. (See Drug Interactions.) TheraCys should not be used in individuals with concurrent infections.
In patients with small bladder capacity, increased risk of bladder contracture should be considered in decisions to treat with TheraCys.
BCG infection of aneurysms and prosthetic devices (including arterial grafts, cardiac devices and artificial joints) have been reported following intravesical administration of BCG. (7) (8) The risk of these ectopic BCG infections has not been determined. The benefits of BCG therapy must be carefully weighed against the possibility of an ectopic BCG infection in patients with pre-existing arterial aneurysms or prosthetic devices of any kind.
If a bacterial urinary tract infection (UTI) occurs during the course of TheraCys treatment, TheraCys instillation should be withheld until complete resolution of the bacterial UTI for two reasons: • the combination of a UTI and BCG-induced cystitis may lead to more severe adverse effects on the genitourinary tract and • BCG bacilli are sensitive to a wide variety of antibiotics; (9) antimicrobial administration may therefore diminish the efficacy of TheraCys. Similarly, patients undergoing antimicrobial therapy for other infections should be evaluated to assess whether the therapy might diminish the efficacy of TheraCys.
The stopper of the vial for this product contains natural rubber latex which may cause allergic reactions.
Management of Serious BCG Complications
Acute, localized irritative toxicities of TheraCys may be accompanied by systemic manifestations, consistent with a "flu-like" syndrome. Systemic adverse effects of 1-2 days' duration such as malaise, fever and chills often reflect hypersensitivity reactions. However, symptoms such as fever of ≥101.3°F (38.5°C ), or acute localized inflammation such as epididymitis, prostatitis, or orchitis persisting longer than 2-3 days suggest active infection and evaluation for serious infectious complications should be considered.
In patients who develop persistent fever or experience an acute febrile illness consistent with BCG infection, two or more antimycobacterial agents should be administered while diagnostic evaluation, including cultures, is conducted. BCG treatment should be discontinued. Negative cultures do not necessarily rule out infection. Physicians using this product should be familiar with the literature on prevention, diagnosis and treatment of BCG-related complications and, when appropriate, should consult an infectious disease specialist or other physician with experience in the diagnosis and treatment of mycobacterial infections.
TheraCys is sensitive to the most commonly used antimycobacterial agents (isoniazid, rifampin and ethambutol). TheraCys is not sensitive to pyrazinamide.
PRECAUTIONS
General
TheraCys contains live mycobacteria and should be prepared and handled using aseptic technique. (See DOSAGE AND ADMINISTRATION, Preparation of Theracys.) BCG infections have been reported in health care workers preparing BCG for administration. Needle stick injuries should be avoided during the handling and mixing of TheraCys.
Nosocomial infections have been reported in immunosuppressed patients receiving parenteral drugs that were prepared in areas in which BCG was prepared. (10) (11)
BCG is capable of dissemination when administered by intravesical route and serious infections, including fatal infections, have been reported in patients receiving intravesical BCG. (6) Care should be taken not to traumatize the urinary tract or to introduce contaminants into the urinary system. Seven to 14 days should elapse before TheraCys is administered following TUR, biopsy, or traumatic catheterization.
TheraCys may be administered to persons in groups at high risk for HIV infection only after careful evaluation of risk/benefit and with extra caution.
The use of TheraCys may cause a falsely positive tuberculin reaction sensitivity. It may therefore be advisable to test to determine the true tuberculin reactivity before treatment. (5)
Information For Patients
TheraCys is retained in the bladder for as long as possible up to 2 hours and then voided. To avoid transmission of BCG to others, for 6 hours after treatment, patients should void while seated in order to avoid splashing of urine. Urine voided during this time should be disinfected with an equal volume of household bleach (5% hypochlorite solution) and allowed to stand for 15 minutes before flushing or disposal. Unless medically contraindicated, patients should be instructed to increase fluid intake in order to "flush" the bladder for several hours following treatment with TheraCys. Patients may experience burning with the first void after treatment.
Because TheraCys contains live mycobacteria, excreted urine may also contain live bacteria. Patients should be advised on appropriate infection control procedures to protect family and close contacts from infection.
Fever, chills, malaise, flu-like symptoms, increased fatigue or an increase in urinary symptoms, (such as burning or pain on urination) are not uncommon. However, patients should notify their physicians if any of these symptoms last more than 48 hours or increase in severity. Patients should also notify their physicians if they experience any of the following: an increase in urinary symptoms (such as urgency, frequency of urination, blood in urine), joint pain, eye complaints (such as pain, irritation or redness), cough, skin rash, jaundice or vomiting.
Drug Interactions
Treatments using immunosuppressants and/myelosuppressants and/or radiation interfere with the development of the immune response to TheraCys and increase the risk of disseminated BCG infection.
Antimicrobial therapy for other infections may interfere with the effectiveness of TheraCys. (12)
As there are no data to suggest that the acute, local urinary tract symptoms common with intravesical BCG are due to mycobacterial infection, antimycobacterial drugs (eg, isoniazid) should not be used prophylactically to prevent the local, irritative side effects of TheraCys.
For patients with a condition that may in the future require mandatory immunosuppression (eg, awaiting an organ transplant, myasthenia gravis) the decision to treat with TheraCys should be considered carefully.
Pregnancy Category C
Animal reproduction studies have not been conducted with TheraCys. It is also not known whether TheraCys can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. TheraCys should not be given to a pregnant woman unless clearly needed. Women should be advised not to become pregnant while on therapy.
Nursing Mothers
It is not known whether TheraCys can be excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from TheraCys in nursing infants, it is advisable to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE EVENTS
Data from Clinical Studies
Administration of TheraCys causes an inflammatory response in the bladder and has been frequently associated with transient fever, hematuria, urinary frequency and dysuria; careful patient monitoring is required. Symptoms of bladder irritability are reported in approximately 50% of patients receiving TheraCys and typically begin 4-6 hours after instillation and last 24-72 hours. The irritative side effects are usually seen following the third instillation and tend to increase in severity after each administration. The mechanism of action of the irritative side effects has not been studied, but is most consistent with an immunological mechanism. There is no evidence that dose reduction or antituberculous drug therapy can prevent or lessen the irritative symptoms of TheraCys.
The adverse reactions which occurred among 127 recipients of TheraCys during a US clinical trial are listed in Table 2. (4) The adverse reactions are combined totals caused by both the original bladder cancer and the TheraCys treatment.
| Adverse Event | Percent of Patients (N = 127) Overall (Grade ≥3) | Adverse Event | Percent of Patients (N = 127) Overall (Grade ≥3) |
|---|---|---|---|
| Dysuria | 52% (4%) | Nausea/Vomiting | 16% (0%) |
| Urinary Frequency | 40% (2%) | Anorexia | 11% (0%) |
| Malaise | 40% (2%) | Renal Toxicity (NOS) | 10% (2%) |
| Hematuria | 39% (7%) | Genital Pain | 10% (0%) |
| Fever (>38°C) | 38% (3%) | Arthralgia/Myalgia | 7% (1%) |
| Chills | 34% (3%) | Urinary Incontinence | 6% (0%) |
| Cystitis | 29% (0%) | Cramps/Pain | 6% (0%) |
| Anemia | 21% (0%) | Diarrhea | 6% (0%) |
| Urinary Tract Infection | 18% (1%) | Contracted Bladder | 5% (0%) |
| Urgency | 18% (0%) | Leukopenia | 5% (0%) |
The following adverse reactions were reported in <5% of patients: coagulopathy, abdominal pain, liver involvement, systemic infection, pulmonary infection, cardiac (unclassified), headache, skin rash, tissue in urine, local infection, constipation, dizziness, fatigue, thrombocytopenia, ureteral obstruction and flank pain.
In a US Clinical Trial, 112 patients received TheraCys. The incidence of adverse reactions associated with intravesical TheraCys is given below.
The following adverse events were reported in ≤1% of patients: tissue in urine, local infection, constipation, dizziness, fatigue, thrombocytopenia and flank pain.
In this study, local irritative symptoms were more common with TheraCys than with doxorubicin; however, grade ≥ 3 irritative toxicity was similar, occurring in approximately 2-7% of patients. Systemic symptoms (fever, chills, malaise, etc.) were also more common with TheraCys. Overall, grade ≥ 3 toxicities were seen in 26 patients (23%) treated with TheraCys and 25 patients (21%) treated with doxorubicin. "Systemic infection" was reported to occur in three patients treated with TheraCys (one grade 2 and two grade 3) and one patient treated with doxorubicin (grade 2). In four patients, treatment was discontinued because of toxicity (two with irritative symptoms, one with severe hematuria and one with possible BCG infection). In addition, six patients refused further treatment because of severe local toxicity and/or chills. Six of these ten patients received TheraCys. Table 3 compares the common adverse events reported in this study.
| Study Arm | ||||
|---|---|---|---|---|
| TheraCys (N=112) | Doxorubicin (N=119) | |||
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| Dysuria | 58 (52%) | 4 (4%) | 48 (40%) | 7 (6%) |
| Frequency | 45 (40%) | 2 (2%) | 34 (29%) | 5 (4%) |
| Malaise | 45 (40%) | 2 (2%) | 17 (14%) | 0 |
| Hematuria | 44 (39%) | 8 (7%) | 33 (28%) | 8 (7%) |
| Fever (>38°C) | 43 (38%) | 3 (3%) | 11 (9%) | 0 |
| Chills | 38 (34%) | 3 (3%) | 7 (6%) | 0 |
| Cystitis | 33 (29%) | 0 | 23 (19%) | 1 (<1%) |
| Urgency | 20 (18%) | 1 (<1%) | 14 (12%) | 3 (2%) |
| Nausea/Vomiting | 18 (16%) | 0 | 10 (8%) | 1 (<1%) |
| Bladder Cramps/Pain | 7 (6%) | 0 | 6 (5%) | 2 (1%) |
Acute, localized irritative side effects of TheraCys may be accompanied by systemic manifestations consistent with a "flu-like" syndrome. Systemic adverse effects of 1-2 days' duration such as malaise, fever and chills often reflect hypersensitivity reactions. However, symptoms such as fever of ≥38.5°C, or acute localized inflammation such as epididymitis, prostatitis, or orchitis, persisting longer than 48 hours suggest active infection and evaluation for serious infectious complication should be considered.
Data from Postmarketing Experience
These events were reported voluntarily from a population of uncertain size, it is not possible to reliably calculate their frequencies.
Symptomatic granulomatous prostatitis, epididymo-orchitis, and renal abscess associated with administration of intravesical BCG have been reported.
Ocular symptoms (including uveitis, conjunctivitis, iritis, keratitis, granulomatous choreoretinitis) alone, or in combination with joint symptoms (arthritis or arthralgia), urinary symptoms and/or skin rash, have been reported following administration of intravesical BCG. The risk appears to be elevated among patients who are positive for HLA-B27. (13)
Skin rash, arthralgia and migratory arthritis may be allergic reactions.
Serious infectious complications of intravesical BCG have been reported. The most serious infectious complication of BCG is disseminated sepsis associated with death. In addition, BCG infections have been reported in eye, lung, liver, bone, bone marrow, kidney, regional lymph nodes, peritoneum and prostate in patients who have received intravesical BCG. Some male genitourinary tract infections (orchitis/epididymitis) have been refractory to multiple drug antimycobacterial therapy and required orchiectomy.
Treatment of Adverse Reactions
If a patient develops persistent fever or experiences an acute febrile illness consistent with BCG infection, BCG instillations should be permanently discontinued, the patient immediately evaluated and treated for BCG infection and an infectious disease consultation sought. (See WARNINGS and PRECAUTIONS.) Treatment with two or more antimycobacterial agents should be initiated promptly while diagnostic evaluation, including cultures, is conducted. Negative cultures do not necessarily rule out infection. TheraCys is sensitive to the most commonly used antimycobacterial agents (isoniazid, rifampin and ethambutol). TheraCys is not sensitive to pyrazinamide. (14)
Reporting of Adverse Reactions
Patients should be encouraged to report all adverse events after treatment with TheraCys. Adverse events should be reported by health care providers to MEDWATCH (call 1-800-FDA-1088 or report on line to www.fda.gov/medwatch). Health care providers should report adverse occurrences temporally related to the administration of the product to the Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater PA 18370 or call 1-800-822-2463.
OVERDOSAGE
Overdosage occurs if more than one vial of TheraCys is administered per instillation. If overdosage occurs, the patient should be closely monitored for signs of active local or systemic infection (See WARNINGS and PRECAUTIONS). For acute local or systemic reactions suggesting active infection, an infectious disease specialist experienced in BCG complications should be consulted.
DOSAGE AND ADMINISTRATION
One dose of TheraCys, BCG Live (Intravesical) consists of the intravesical instillation of 81 mg (dry weight) BCG. (See Preparation of TheraCys.)
For intravesical instillation only. Do not inject subcutaneously or intravenously.
A urethral catheter is inserted into the bladder under aseptic conditions, the bladder is drained and then 50 mL suspension of TheraCys is instilled slowly by gravity, following which the catheter is withdrawn.
The patient retains the suspension for as long as possible for a total of up to two hours. During the first 15 minutes following instillation, the patient should lie prone. Thereafter, the patient is allowed to be up. At the end of 2 hours, all patients should void in a seated position for safety reasons. Patients should be instructed to increase fluid intake in order to flush the bladder in the hours following BCG treatment.
Preparation of TheraCys
The preparation of the TheraCys suspension should be done using aseptic technique. To avoid cross-contamination, parenteral drugs should not be prepared in areas where BCG has been prepared. A separate area for the preparation of the TheraCys suspension is strongly recommended. All equipment, supplies and receptacles in contact with TheraCys should be handled and disposed of as biohazardous. The individual responsible for mixing the agent should wear gloves and eye protection and take precautions to avoid contact of BCG with broken skin. If the preparation cannot be performed in a biocontainment hood, then a mask and gown may be worn to avoid inhalation of BCG organisms and inadvertent exposure to broken skin.
TheraCys should not be handled by persons with an immunodeficiency.
- Do not remove the rubber stopper from the vial.
- Cleanse the surface of the rubber stopper of the vial of TheraCys with a suitable antiseptic.
- Using a 5 mL sterile syringe and needle, draw up 3 mL of sterile preservative-free saline solution.
- Using the same syringe and needle, pierce the rubber stopper in the vial of freeze-dried material with the needle.
- Hold the vial of freeze-dried material upright and pull the plunger of the syringe back to create a mild vacuum in the vial.
- Release the plunger and allow the vacuum to pull the saline from the syringe into the vial of freeze-dried material. After all the saline has passed into the freeze-dried material, remove the needle and syringe.
- Shake the vial gently until a fine, even suspension results. Avoid foaming since this will prevent withdrawal of the proper dose.
- Withdraw the entire contents (approximately 3 mL) of the reconstituted material into the syringe. Return the vial to an upright position before removing the syringe from the vial.
- Further dilute the reconstituted material from the vial (1 dose) in sterile, preservative-free saline to a final volume of 50 mL for intravesical instillation.
- TheraCys should be used immediately after reconstitution. However, if there is an unavoidable delay between reconstitution and administration, this delay must not exceed 2 hours at a temperature between 2° and 25°C (35° and 77°F).
Any reconstituted product which exhibits flocculation or clumping that cannot be dispersed with gentle shaking should not be used.
Reconstituted product should not be exposed to sunlight, direct or indirect. Exposure to artificial light should be kept to a minimum.
Instructions for Disposal
After use, unused product, packaging and all equipment and materials used for instillation of the product (eg, syringes, catheters) should be placed immediately in a container for biohazardous materials and disposed of according to local requirements applicable to biohazardous materials.
Urine voided during the 6 hour period following TheraCys instillation should be disinfected with an equal volume of 5% hypochlorite solution (undiluted household bleach) and allowed to stand for 15 minutes before flushing. (See PRECAUTIONS, Information For Patients.)
Treatment Schedule
Intravesical treatment of the urinary bladder should begin 7 to 14 days after biopsy or transurethral resection and consists of induction and maintenance therapy. For the induction therapy, one dose of TheraCys is administered each week for 6 consecutive weeks. Induction therapy should be followed by maintenance therapy, consisting of one dose given 3, 6, 12, 18 and 24 months following the initial dose.
HOW SUPPLIED
Vial, 1 dose of the freeze dried product (1 per package) - NDC No. 49281-880-03.
STORAGE
TheraCys, BCG Live (Intravesical) should be stored at 2° to 8 °C (35° to 46 °F). Do not use beyond the expiration date, otherwise it may be inactive.
At no time should the freeze-dried TheraCys be exposed to sunlight, direct or indirect. Exposure to artificial light should be kept to a minimum.
REFERENCES
- 1
- Mikkelsen DJ, Ratliff TL. Mechanisms of action of intravesical Bacillus Calmette-Guérin for bladder cancer. In: Urologic Oncology. 1989:195-211.
- 2
- O'Donnell MA, DeWolf WC. BCG immunotherapy for superficial bladder cancer. New prospects for an old warhorse. Surg Oncol Clin North Amer 1995;4:189-202.
- 3
- Prescott S, et al. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol 1992:147:1636-42.
- 4
- Lamm DL, et al. A randomized trial of intravesical doxorubicin and immunotherapy with Bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Eng J Med 1991;325:1205-9.
- 5
- CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR 2005;54(RR-17):1-89.
- 6
- Lamm DL, et al. Incidence and treatment of complications of Bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol 1992;147:596-600.
- 7
- Stone D. Mycobacterium bovis infection of an implantable defibrillator following intravesical therapy with bacille Calmette-Guérin. Clin Inf Dis 1993;16:825-6.
- 8
- Wolf YG, et al. Infection of a ruptured aortic aneurysm and an aortic graft with bacille Calmette-Guérin after intravesical administration for bladder cancer. J Vasc Surg 1995;22:80-4.
- 9
- Durek C, et al. Interference of modern antibacterials with bacillus Calmette-Guérin viability. J Urol 1999;162:1959-62.
- 10
- Stone MM, et al. Brief report: Meningitis due to iatrogenic BCG infection in two immunocompromised children. N Engl J Med 1995,333:561-3.
- 11
- Waecker NJ, et al. Nosocomial transmission of Mycobacterium bovis bacille Calmette-Guérin to children receiving cancer therapy and to their health-care providers. Clin Infect Dis 2000;30:356-62.
- 12
- Böhle A, Jocham D. Intravesical immunotherapy with Bacillus Calmette-Guérin, facts, figures and results. München, Germany: Urban & Fischer; 2000. p. 111-2.
- 13
- Wittes RC, et al. Characterization of BCG-associated sterile arthritis and Reiter's syndrome. New Delhi, India: 10th International Congress of Immunology. 1998:1245-9.
- 14
- van der Meijden APM, et al. The possible influence of antibiotics on results of Bacillus Calmette-Guérin intravesical therapy for superficial bladder cancer. J Urol 1991;146:444-6.
Product Information as of July 2011.
Printed in Canada
Manufactured by:
Sanofi Pasteur Limited
Toronto Ontario Canada
Distributed by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
R6-0711 USA
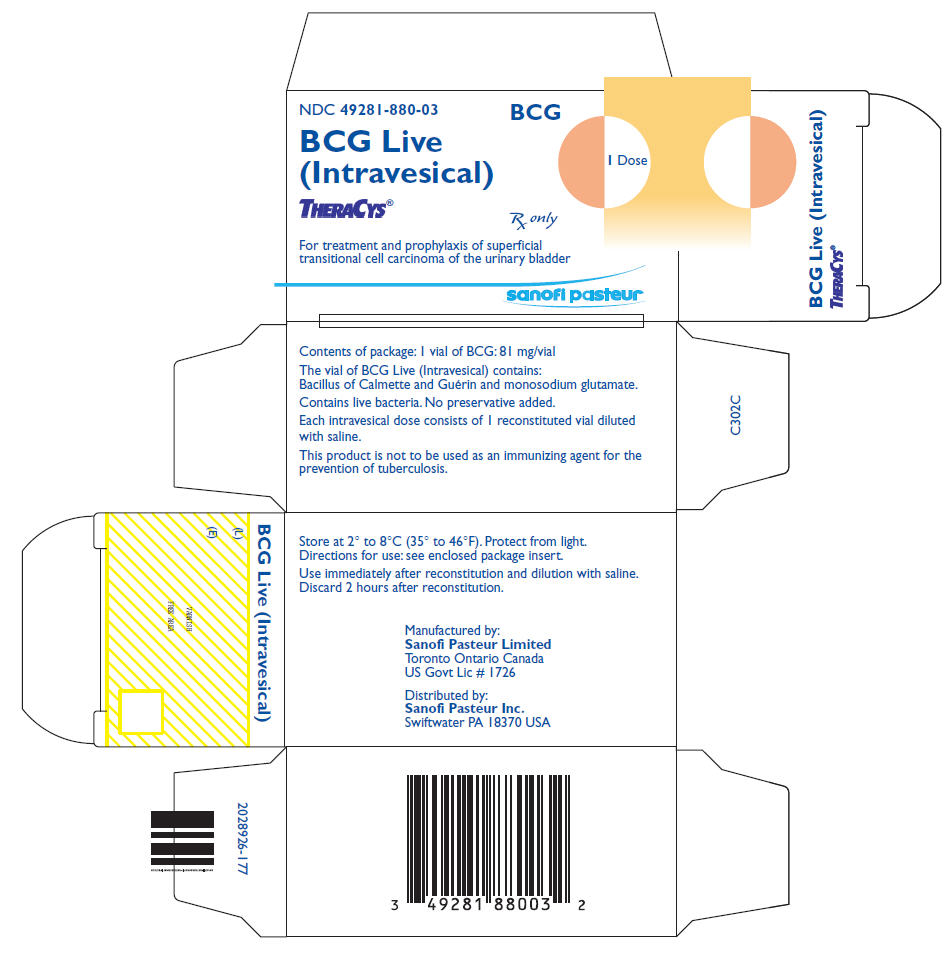
PRINCIPAL DISPLAY PANEL - 81 mg Vial Label
NDC 49281-880-03
BCG Live (Intravesical)
THERACYS®
81 mg. Contains live bacteria.
Store at 2° to 8°C (35° to 46°F).
Protect from light. Reconstitute for use.
Sanofi Pasteur Limited
BCG
1 Dose
Rx only

PRINCIPAL DISPLAY PANEL - 81 mg Vial Carton (No Diluent)
NDC 49281-880-03
BCG Live
(Intravesical)
THERACYS®
For treatment and prophylaxis of superficial
transitional cell carcinoma of the urinary bladder
BCG
1 Dose
Rx only
sanofi pasteur
| THERACYS
bacillus calmette-guerin substrain connaught live antigen injection, powder, lyophilized, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103943 | 07/22/2011 | |
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sanofi Pasteur Limited | 208206623 | MANUFACTURE | |
Revised: 09/2011 Sanofi Pasteur Inc.