EQUALIZER GAS RELIEF
-
dimethicone suspension/ drops
Hi-Tech Pharmacal Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Directions
- shake well before each use
- administer dose 4 times daily after meals and at bedtime. The dosage can also be mixed with 1 oz of cool water, infant formula or other suitable liquid to ease administration.
- do not give more than 12 doses in 24 hours
- infants under 2 years (under 24 lbs) 0.3 mL
- children over 2 years (over 24 lbs) 0.6 mL
Inactive ingredients
Calcium saccharin, carbopol 934P, citric acid, D&C Red #33, hydroxypropylmethylcellulose, purified water, sodium benzoate, vanillin. Sodium citrate may be used to adjust pH.
Questions or comments?
Call 1-800-262-9010, Mon.-Thurs. 9:00 am - 4:30 pm EST, Fri, 9:00 am - 2:30 pm EST. Serious side effects associated with use of this product may be reported to this number.
Rev. 785:01 12/08
Package/Label Principal Display Panel
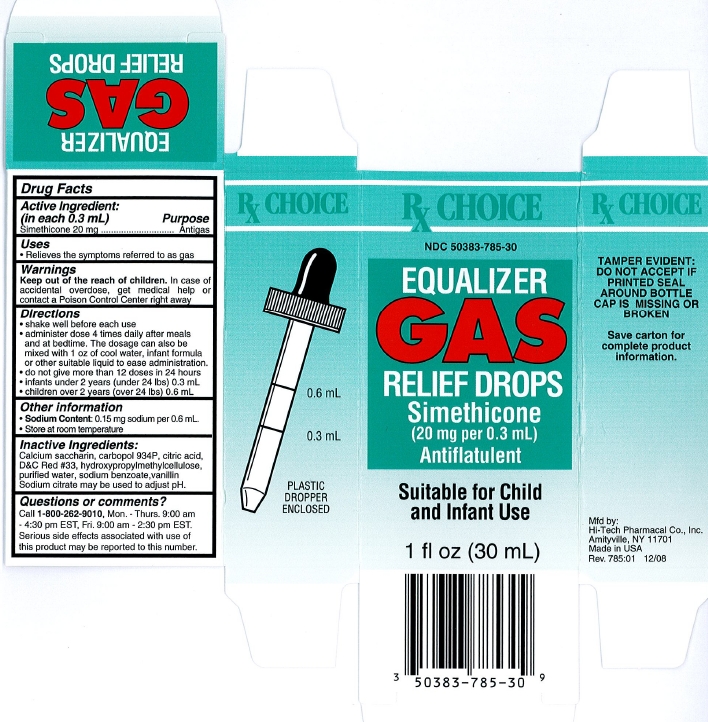
NDC 50383-785-30
EQUALIZER GAS RELIEF DROPS
Simethicone
(20 mg per 0.3 mL)
Antiflatulent
Suitable for Child and Infant Use
1 fl oz (30 mL)
| EQUALIZER GAS RELIEF
simethicone suspension/ drops |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part332 | 10/01/1991 | |
| Labeler - Hi-Tech Pharmacal Co., Inc. (101196749) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Hi-Tech Pharmacal Co., Inc. | 101196749 | MANUFACTURE | |
Revised: 08/2011 Hi-Tech Pharmacal Co., Inc.