GYNE-SULF
-
sulfathiazole,
sulfacetamide and
sulfabenzamide cream
GW Laboratories, Inc.
----------
DESCRIPTION
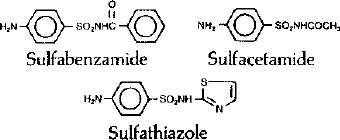
Triple Sulfa Vaginal Cream, USP is a topical antibacterial preparation available for intravaginal administration containing the following active ingredients: sulfathiazole (Benzenesulfonamide, 4-amino-N-2-thiazolyl-N12-thiazolylsulfanilanlide) 3.42%, sulfucetamide (Acetamide,N-[(4-aminophenyl)sulfonyl]-N-Sulfanilylacetamide) 2.86%, sulfabenzamide (Benzamide,N-[(4-aminophenyl) sulfonyl]-N-Sulfanilylbenzamide)3.7% and urea (carbamide) 0.64 % in a base consisting of glycerol monostearate, propylene glycol, cetyl alcohol, peanut oil, stearic acid, lanolin, lecithin, diethylaminoethyl stearamide, cholesterol, phosphoric acid, methylparaben, propylparaben, butylated hydroxytoluene and purified water. pH range 3.0 to 4.0. pH adjusted with additional phosphoric acid if necessary.
CLINICAL PHARMACOLOGY
The mode of action of Triple Sulfa Vaginal Cream is not completely known. Triple Sulfa Vaginal Cream is a topical antibacterial preparation used intravaginally against Haemophilus(Gardnerella) vaginalis bacteria. Indirect effects, such as lowering the vaginal pH, may be equally important mechanisms.
INDICATIONS & USAGE
Triple Sulfa Vaginal Cream is indicated for the treatment of vaginitis caused by Haemophilus (Gardnerella) vaginalis bacteria.
The diagnosis of a Haemophilus (Gardnerella) vaginalis vaginitis should be firmly established before initiation of treatment with Triple Sulfa Vaginal Cream.
CONTRAINDICATIONS
Triple Sulfa Vaginal Cream is contraindicated in the following circumstances: kidney disease; hypersensitivity to sulfonamides; in pregnancy at term and during the nursing period because sulfonamides cross the placenta, are excreted in breast milk and may cause kernicterus.
WARNINGS
Deaths associated with the administration of sulfonamides have been reported from hypersensitivity reactions, agranulocytosis, aplastic anemia and other blood dyscrasias.
The presence of clinical signs such as sore throat, fever, pallor, purpura or jaundice may be early indications of serious blood disorders.
PRECAUTIONS
Because sulfonamides may be absorbed from the vaginal mucosa, the usual precautions for oral sulfonamides apply. Patients should be observed for skin rash or evidence of systemic toxicity, and if these develop, the medication should be discontinued.
Laboratory Tests: Standard office diagnostic procedures for vaginitis are usually sufficient to establish the diagnosis of Haemophilus (Gardnerella) vaginalis and to rule out a trichomonal or monilial infection, These include noting a fish-like odor upon addition of 10%potassium hydroxide to vaginal discharge and microscopic identification of "clue cells" in a wet mount preparation. If cultures are obtained, care must be taken to use appropriate media and methods for Haemophilus (Gardnerella) vaginalis.
Carcinogenesis, Mutagenesis, Impairment of Fertility: The sulfonamides bear certain chemical similarity to some goitrogens. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides, and long-term administration has produced thyroid malignancies in this species.
Pregnancy:
Teratogenic Effects: Pregnancy Category C: The safe use of sulfonamides in pregnancy has not been established.The teratogenicity potential of most sulfonamides has not been thoroughly investigated in either animals or humans. However, a significant increase in the incidence of cleft palate and other bony abnormalities of offspring has been observed when certain sulfonamides of the short, intermediate and long-acting types were given to pregnant rats and mice at high oral doses (7 to 25 times the human therapeutic dose).
Nursing Mothers: Because of the potential for serious adverse reactions in nursing infants from Triple Sulfa Vaginal Cream. a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. See CONTRAlNDlCATIONS
Pediatric Use: Safety and effectiveness in children have not been established.
ADVERSE REACTIONS
There has been one reported case of agranulocytosis in a patient receiving a triple sulfa vaginal cream, The most frequent adverse reactions to Triple Sulfa Vaginal Cream are localized irritation and/or allergy including rare reports of Stevens Johnson syndrome which may be fatal.
DOSAGE & ADMINISTRATION
One applicatorful intravaginally twice daily for 4 to 6 days. This course of therapy may be repeated if necessary; the dosage may be reduced one-half to one-quarter.
HOW SUPPLIED
Triple Sulfa Vaginal Cream is available in 2.75 oz (78 g) tubes complete with measured-dose applicator. One applicatorful contains approximately 5 grams.
SPL PATIENT PACKAGE INSERT
Patient Information
Gardnerella (Haemophilus)
Vaginalis Vaginitis
This information leaflet is designed to help you understand your vaginal infection. It is not intended to replace or to contradict your physician's instructions.
INTRODUCTION: Infection of the vagina (vaginitis) is usually caused by three different kinds of microscopic organisms: 1) Trichomonas - a protozoan, 2) Candida- a yeast, and 3) Gardnerella (Haemophilus) - a bacterium.
The medical treatment varies with the infective agent. When more than one organism is responsible, the condition may be called "mixed vaginitis". Your doctor has prescribed Triple Sulfa Vaginal Cream. It is effective in treating vaginitis when Gardnerella (Haemophilus) is causing the infection.
HOW DID I GET THIS INFECTION? The exact cause of the infection is a subject of some controversy. Natural body estrogens stimulates the vagina to product the nutrients that support the growth of Gardnerella vaginalis (Haemophilus). The infection can be transmitted by sexual intercourse or by inanimate objects such as as douches nozzles, bath towels, or wet bathing suits.
EXACTLY WHERE IS THE INFECTION? Gardnerella (Haemophilus) us called a "surface parasite" because it lives on the cells of the vaginal surface, and because it does not usually invade more deeply into the tissues.
WHAT ARE THE CHARACTERISTICS OF GARDNERELLA (HAEMOPHILUS) VAGINITIS? The symptoms usually are (1) excessive vaginal discharge, and (2) odor. A few patients with this condition will also have vaginal itching, burning, or irritations, but most do not.
The vaginal discharge of Gardnerella (Haemophilus) vaginitis is usually grey or yellow and like a "thin flour paste". The odor is often described as "fishy" and is felt by many patients to be the most disagreeable symptoms of the infection. There is usually no swelling or redness of the vaginal area.
WHAT IS A MEDICAL DIAGNOSIS OF GARDNERELLA (HAEMOPHILUS) VAGINITIS MADE? The diagnosis of Gardnerella (Haemophilus) vaginitis can be established clinically by the symptoms you report, and by the finding on pelvic (internal) examination.
The health professional can examine a small amount of vaginal discharge under the microscope, and large amounts of the typical bacteria can be seen in and around many vaginal cells in the discharge. If in addition a microscopic slide is stained appropriately, the Gardnerella bacteria can be identified with a higher degree of confidence.
DOES GARDNERELLA (HAEMOPHILUS) CAUSE INFECTION IN MALES? The male can carry Gardnerella in his genital tract. Sometimes symptoms develop,but usually less often than in the female. Because the male can be a source of reinfection n the female, some physician prefer to treat the sexual partner with oral tablets while the female is being treated. Triple Sulfa Vaginal Cream is indicated for local therapy and is not used for oral treatment. The use of condoms my be helpful in avoiding reinfection.
G & W Laboratories, Inc.
South Plainfield, NJ 07080
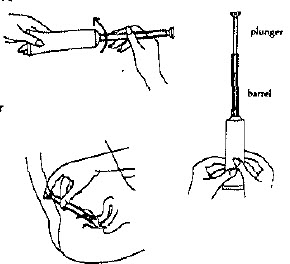
DIRECTIONS FOR USING THE VAGINAL APPLICATOR
Filling the applicator
Remove the cap from tube. Screw applicator onto tube.
Squeeze tube forcing contents into barrel until it is full, Then remove applicator from tube.
Lie on your back with knees drawn up, Hold filled applicator by barrel and gently insert in into the vagina as far as it will go comfortably. Press plunger and deposit material. While keeping plunger depressed, remove the applicator from vagina.
Care of the applicator
After each use, pull applicator apart and wash with soap and warm water.
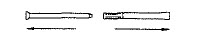
To assemble, gently push plunger back into barrel as far as it will go.
NOTE: Store at room temperature (59°- 86°F).
PACKAGE LABEL
NDC 0713-0214-33
GYNE-SULF TM Triple Sulfa Vaginal Cream, USP with Applicator
Caution: Federal law prohibits dispensing without prescription.
Store at controlled room temperature, 15°-30°C (59°-86°F). Net Weight: 2.75 oz (78g)
Composition: Sulfathiazole 3.42%, Sulfacetamide 2.86%, Sulfabenzamide 3.7%, Urea 0.64%, in a cream base consisting of Glycerol Monostearate, Propylene Glycol, Cetyl Alcohol, Peanut Oil, Stearic Acid, Lanolin, Lecithin, Diethylaminoethyl Stearamide, Cholesterol, Phosphoric Acid, Methylparaben, Propylparaben, Butylated Hydroxyltoluene, and Purified Water. pH range 3.0 to 4.0 pH adjusted with additional Phosphoric Acid, if necessary.
Warning: Keep this and all drugs out of the reach of children. In case of accidental ingestion, seek professional assistance or contact a poison control center immediately.
IMPORTANT PATIENT INFORMATION LEAFLET INSIDE
Pharmacist Please Note The Combined Insert
Unless otherwise instructed by the Physician, dispense prescription with Patient Package insert only. Remove physician Package Insert at perforation.
G&W Laboratories, Inc.
South Plainfield, NJ 07080
REV. 3/90

| GYNE-SULF
triple sulfa vaginal cream cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA088607 | 08/03/1983 | 09/01/1995 |
| Labeler - GW Laboratories, Inc. (001271188) |
| Registrant - GW Laboratories, Inc. (001271188) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GW Laboratories, Inc. | 001271188 | MANUFACTURE | |
Revised: 09/2011 GW Laboratories, Inc.