bicnu (CARMUSTINE)
[Bristol-Myers Squibb]
WARNINGS
BiCNU (carmustine for injection) should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents.
Bone marrow suppression, notably thrombocytopenia and leukopenia, which may contribute to bleeding and overwhelming infections in an already compromised patient, is the most common and severe of the toxic effects of BiCNU (see WARNINGS and ADVERSE REACTIONS).
Since the major toxicity is delayed bone marrow suppression, blood counts should be monitored weekly for at least 6 weeks after a dose (see ADVERSE REACTIONS). At the recommended dosage, courses of BiCNU should not be given more frequently than every 6 weeks.
The bone marrow toxicity of BiCNU is cumulative and therefore dosage adjustment must be considered on the basis of nadir blood counts from prior dose (see “Dosage Adjustment Table” under DOSAGE AND ADMINISTRATION).
Pulmonary toxicity from BiCNU appears to be dose related. Patients receiving greater than 1400 mg/m2 cumulative dose are at significantly higher risk than those receiving less.
Delayed pulmonary toxicity can occur years after treatment, and can result in death, particularly in patients treated in childhood (see ADVERSE REACTIONS and PRECAUTIONS: Pediatric Use).
DESCRIPTION
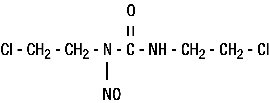
BiCNU® (carmustine for injection) is one of the nitrosoureas used in the treatment of certain neoplastic diseases. It is 1,3-bis (2-chloroethyl)-1-nitrosourea. It is sterile lyophilized pale yellow flakes or congealed mass with a molecular weight of 214.06. It is highly soluble in alcohol and lipids, and poorly soluble in water. BiCNU is administered by intravenous infusion after reconstitution as recommended.
The structural formula is:
BiCNU is available in 100 mg single dose vials of lyophilized material. Sterile diluent for constitution of BiCNU is co-packaged with the active drug product for use in constitution of the lyophile. The diluent is supplied in an ampule containing 3 mL of Dehydrated Alcohol Injection, USP.
CLINICAL PHARMACOLOGY
Although it is generally agreed that carmustine alkylates DNA and RNA, it is not cross-resistant with other alkylators. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins.
Intravenously administered carmustine is rapidly degraded, with no intact drug detectable after 15 minutes. However, in studies with 14C-labeled drug, prolonged levels of the isotope were detected in the plasma and tissue, probably representing radioactive fragments of the parent compound.
It is thought that the antineoplastic and toxic activities of carmustine may be due to metabolites. Approximately 60% to 70% of a total dose is excreted in the urine in 96 hours and about 10% as respiratory CO2. The fate of the remainder is undetermined.
Because of the high lipid solubility and the relative lack of ionization at physiological pH, carmustine crosses the blood-brain barrier quite effectively. Levels of radioactivity in the CSF are ≥50% of those measured concurrently in plasma.
INDICATIONS AND USAGE
BiCNU is indicated as palliative therapy as a single agent or in established combination therapy with other approved chemotherapeutic agents in the following:
- Brain tumors—glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors.
- Multiple myeloma—in combination with prednisone.
- Hodgkin’s Disease—as secondary therapy in combination with other approved drugs in patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy.
- Non-Hodgkin’s lymphomas—as secondary therapy in combination with other approved drugs for patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy.
CONTRAINDICATIONS
BiCNU should not be given to individuals who have demonstrated a previous hypersensitivity to it.
WARNINGS
Since the major toxicity is delayed bone marrow suppression, blood counts should be monitored weekly for at least 6 weeks after a dose (see ADVERSE REACTIONS). At the recommended dosage, courses of BiCNU should not be given more frequently than every 6 weeks.
The bone marrow toxicity of BiCNU is cumulative and therefore dosage adjustment must be considered on the basis of nadir blood counts from prior dose (see “Dosage Adjustment Table” under DOSAGE AND ADMINISTRATION).
Pulmonary toxicity from BiCNU appears to be dose related. Patients receiving greater than 1400 mg/m2 cumulative dose are at significantly higher risk than those receiving less. Additionally delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in patients who receive BiCNU in childhood and early adolescence (see ADVERSE REACTIONS).
Long-term use of nitrosoureas has been reported to be associated with the development of secondary malignancies.
Liver and renal function tests should be monitored periodically (see ADVERSE REACTIONS).
BiCNU (carmustine for injection) may cause fetal harm when administered to a pregnant woman. BiCNU has been shown to be embryotoxic in rats and rabbits and teratogenic in rats when given in doses equivalent to the human dose. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking (receiving) this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
BiCNU has been administered through an intraarterial intracarotid route; this procedure is investigational and has been associated with ocular toxicity.
PRECAUTIONS
General
In all instances where the use of BiCNU is considered for chemotherapy, the physician must evaluate the need and usefulness of the drug against the risks of toxic effects or adverse reactions. Most such adverse reactions are reversible if detected early. When such effects or reactions do occur, the drug should be reduced in dosage or discontinued and appropriate corrective measures should be taken according to the clinical judgment of the physician. Reinstitution of BiCNU therapy should be carried out with caution, and with adequate consideration of the further need for the drug and alertness as to possible recurrence of toxicity.
Laboratory Tests
Due to delayed bone marrow suppression, blood counts should be monitored weekly for at least 6 weeks after a dose.
Baseline pulmonary function studies should be conducted along with frequent pulmonary function tests during treatment. Patients with a baseline below 70% of the predicted Forced Vital Capacity (FVC) or Carbon Monoxide Diffusing Capacity (DLCO) are particularly at risk.
Since BiCNU may cause liver dysfunction, it is recommended that liver function tests be monitored.
Renal function tests should also be monitored periodically.
Drug Interactions
Greater myelotoxicity (e.g., leukopenia and neutropenia) have been reported when carmustine was combined with cimetidine (see ADVERSE REACTIONS: Hematologic Toxicity).
Carcinogenesis, Mutagenesis, Impairment of Fertility
BiCNU is carcinogenic in rats and mice, producing a marked increase in tumor incidence in doses approximating those employed clinically. Nitrosourea therapy does have carcinogenic potential in humans (see ADVERSE REACTIONS). BiCNU also affects fertility in male rats at doses somewhat higher than the human dose.
Pregnancy
Pregnancy Category D
See WARNINGS.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because of the potential for serious adverse events in nursing infants, nursing should be discontinued while taking BiCNU.
Pediatric Use
Safety and effectiveness in children have not been established. Delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in a long-term study of patients who received BiCNU in childhood and early adolescence (1–16 years). Eight out of the 17 patients (47%) who survived childhood brain tumors, including all the five patients initially treated at less than five years of age, died of pulmonary fibrosis. Therefore, the risks and benefits of BiCNU therapy must be carefully considered, due to the extremely high risk of pulmonary toxicity. (See ADVERSE REACTIONS: Pulmonary Toxicity.)
Geriatric Use
No data from clinical studies of BiCNU are available for patients 65 years of age and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dose range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
BiCNU and its metabolites are known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and renal function should be monitored.
ADVERSE REACTIONS
Pulmonary Toxicity
Pulmonary toxicity characterized by pulmonary infiltrates and/or fibrosis has been reported to occur from 9 days to 43 months after treatment with BiCNU and related nitrosoureas. Most of these patients were receiving prolonged therapy with total doses of BiCNU greater than 1400 mg/m2. However, there have been reports of pulmonary fibrosis in patients receiving lower total doses. Other risk factors include past history of lung disease and duration of treatment. Cases of fatal pulmonary toxicity with BiCNU have been reported.
Additionally, delayed onset pulmonary fibrosis occurring up to 17 years after treatment has been reported in a long-term study with 17 patients who received BiCNU in childhood and early adolescence (1–16 years) in cumulative doses ranging from 770 to 1800 mg/m2 combined with cranial radiotherapy for intracranial tumors. Chest x-rays demonstrated pulmonary hypoplasia with upper zone contraction. Gallium scans were normal in all cases. Thoracic CT scans have demonstrated an unusual pattern of upper zone fibrosis. There was some late reduction of pulmonary function in all long-term survivors. This form of lung fibrosis may be slowly progressive and has resulted in death in some cases. In this long-term study, 8 of 17 died of delayed pulmonary lung fibrosis, including all those initially treated (5 of 17) at less than 5 years of age.
Hematologic Toxicity
A frequent and serious toxicity of BiCNU is delayed myelosuppression. It usually occurs 4 to 6 weeks after drug administration and is dose related. Thrombocytopenia occurs at about 4 weeks postadministration and persists for 1 to 2 weeks. Leukopenia occurs at 5 to 6 weeks after a dose of BiCNU and persists for 1 to 2 weeks. Thrombocytopenia is generally more severe than leukopenia. However, both may be dose-limiting toxicities.
BiCNU may produce cumulative myelosuppression, manifested by more depressed indices or longer duration of suppression after repeated doses.
The occurrence of acute leukemia and bone marrow dysplasias have been reported in patients following long-term nitrosourea therapy.
Anemia also occurs, but is less frequent and less severe than thrombocytopenia or leukopenia.
Greater myelotoxicity (e.g., leukopenia and neutropenia) has been reported when carmustine was combined with cimetidine (see PRECAUTIONS: Drug Interactions).
Gastrointestinal Toxicity
Nausea and vomiting after IV administration of BiCNU are noted frequently. This toxicity appears within 2 hours of dosing, usually lasting 4 to 6 hours, and is dose related. Prior administration of antiemetics is effective in diminishing and sometimes preventing this side effect.
Hepatotoxicity
A reversible type of hepatic toxicity, manifested by increased transaminase, alkaline phosphatase and bilirubin levels, has been reported in a small percentage of patients receiving BiCNU.
Nephrotoxicity
Renal abnormalities consisting of progressive azotemia, decrease in kidney size and renal failure have been reported in patients who received large cumulative doses after prolonged therapy with BiCNU and related nitrosoureas. Kidney damage has also been reported occasionally in patients receiving lower total doses.
Other Toxicities
Accidental contact of reconstituted BiCNU with skin has caused burning and hyperpigmentation of the affected areas.
Rapid IV infusion of BiCNU (carmustine for injection) may produce intensive flushing of the skin and suffusion of the conjunctiva within 2 hours, lasting about 4 hours. It is also associated with burning at the site of injection although true thrombosis is rare.
Neuroretinitis, chest pain, headache, allergic reaction, hypotension and tachycardia have been reported as part of ongoing surveillance.
OVERDOSAGE
No proven antidotes have been established for BiCNU overdosage.
DOSAGE AND ADMINISTRATION
The recommended dose of BiCNU as a single agent in previously untreated patients is 150 to 200 mg/m2 intravenously every 6 weeks. This may be given as a single dose or divided into daily injections such as 75 to 100 mg/m2 on 2 successive days. When BiCNU is used in combination with other myelosuppressive drugs or in patients in whom bone marrow reserve is depleted, the doses should be adjusted accordingly.
Doses subsequent to the initial dose should be adjusted according to the hematologic response of the patient to the preceding dose. The following schedule is suggested as a guide to dosage adjustment:
| Nadir After Prior Dose | Percentage
of Prior Dose to be Given |
|
| Leukocytes/mm3 | Platelets/mm3 | |
| >4000 | >100,000 | 100% |
| 3000–3999 | 75,000–99,999 | 100% |
| 2000–2999 | 25,000–74,999 | 70% |
| <2000 | <25,000 | 50% |
A repeat course of BiCNU should not be given until circulating blood elements have returned to acceptable levels (platelets above 100,000/mm3, leukocytes above 4,000/mm3), and this is usually in 6 weeks. Adequate number of neutrophils should be present on a peripheral blood smear. Blood counts should be monitored weekly and repeat courses should not be given before 6 weeks because the hematologic toxicity is delayed and cumulative.
Administration Precautions
As with other potentially toxic compounds, caution should be exercised in handling BiCNU and preparing the solution of BiCNU. Accidental contact of reconstituted BiCNU with the skin has caused transient hyperpigmentation of the affected areas. The use of gloves is recommended. If BiCNU lyophilized material or solution contacts the skin or mucosa, immediately wash the skin or mucosa thoroughly with soap and water.
The reconstituted solution should be used intravenously only and should be administered by IV drip. Injection of BiCNU over shorter periods of time than 1 to 2 hours may produce intense pain and burning at the site of injection.
Preparation of Intravenous Solutions
First, dissolve BiCNU with 3 mL of the supplied sterile diluent (Dehydrated Alcohol Injection, USP). Second, aseptically add 27 mL Sterile Water for Injection, USP. Each mL of resulting solution contains 3.3 mg of BiCNU in 10% ethanol. Such solutions should be protected from light.
Reconstitution as recommended results in a clear, colorless to yellowish solution which may be further diluted with 5% Dextrose Injection, USP. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Important Note
The lyophilized dosage formulation contains no preservatives and is not intended for use as a multiple dose vial.
Stability
The unopened vial of the dry drug must be stored in a refrigerator (2°-8°C, 36°-46°F). The diluent ampules may be stored at controlled room temperature (59°-86°F, 15°-30°C) or in a refrigerator (2°-8°C, 36°-46°F). The recommended storage of unopened BiCNU vials provides a stable product for up to 3 years. After reconstitution as recommended, BiCNU is stable for 24 hours under refrigeration (2°-8°C, 36°-46°F). Reconstituted vials should be examined for crystal formation prior to use. If crystals are observed, they may be redissolved by warming the vial to room temperature with agitation.
Vials reconstituted as directed and further diluted to a concentration of 0.2 mg/mL in 5% Dextrose Injection, USP, should be stored at room temperature, protected from light and utilized within 8 hours.
Glass containers were used for the stability data provided in this section. Only use glass containers for BiCNU administration.
Important Note
BiCNU has a low melting point (30.5°-32.0°C or 86.9°-89.6°F). Exposure of the drug to this temperature or above will cause the drug to liquefy and appear as an oil film on the vials. This is a sign of decomposition and vials should be discarded. If there is a question of adequate refrigeration upon receipt of this product, immediately inspect the vial in each individual carton. Hold the vial to a bright light for inspection. The BiCNU will appear as a very small amount of dry flakes or dry congealed mass. If this is evident, the BiCNU is suitable for use and should be refrigerated immediately.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
To minimize the risk of dermal exposure, always wear impervious gloves when handling vials containing BiCNU. This includes all handling activities in clinical settings, pharmacies, storerooms, and home healthcare settings, including during unpacking and inspection, transport within a facility, and dose preparation and administration.
HOW SUPPLIED
BiCNU® (carmustine for injection). Each package includes a vial containing 100 mg carmustine and an ampule containing 3 mL sterile diluent.
NDC 0015-3012-60
STORAGE
Store in a refrigerator (2°-8°C, 36°-46°F).
Store diluent at controlled room temperature (59°-86°F, 15°-30°C) or in a refrigerator (2°-8°C, 36°-46°F).
REFERENCES
- ONS Clinical Practice Committee. Cancer Chemotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society; 1999:32-41.
- Recommendations for the safe handling of cytotoxic drugs. Washington, DC: Division of Safety, Clinical Center Pharmacy Department and Cancer Nursing Services, National Institutes of Health; 1992. US Dept of Health and Human Services, Public Health Service Publication NIH 92-2621.
- AMA Council on Scientific Affairs. Guidelines for handling parenteral antineoplastics. JAMA. 1985;253:1590-1592.
- National Study Commission on Cytotoxic Exposure. Recommendations for handling cytotoxic agents. 1987. Available from Louis P. Jeffrey, ScD, Chairman, National Study Commission on Cytotoxic Exposure. Massachusetts College of Pharmacy and Allied Health Sciences, 179 Longwood Avenue, Boston, MA 02115.
- Clinical Oncological Society of Australia. Guidelines and recommendations for safe handling of antineoplastic agents. Med J Aust. 1983;1:426-428.
- Jones RB, Frank R, Mass T. Safe handling of chemotherapeutic agents: a report from The Mount Sinai Medical Center. CA Cancer J Clin. 1983;33:258-263.
- American Society of Hospital Pharmacists. ASHP technical assistance bulletin on handling cytotoxic and hazardous drugs. Am J Hosp Pharm. 1990;47:1033-1049.
- Controlling occupational exposure to hazardous drugs. (OSHA Work-Practice Guidelines.) Am J Health-Syst Pharm. 1996;53:1669-1685.
BiCNU manufactured by:
Ben Venue Laboratories,
Inc.
Bedford, OH 44146
Diluent manufactured by:
Luitpold
Pharmaceuticals, Inc.
Shirley, NY 11967
Distributed
by:
Bristol-Myers Squibb Company
Princeton, NJ 08543
USA
51-032517-01
1223678A1
Rev August 2007
| BICNU (CARMUSTINE) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 10/2007Bristol-Myers Squibb