BENZPHETAMINE HYDROCHLORIDE
-
benzphetamine hydrochloride tablet
PD-Rx Pharmaceuticals, Inc.
----------
DESCRIPTION
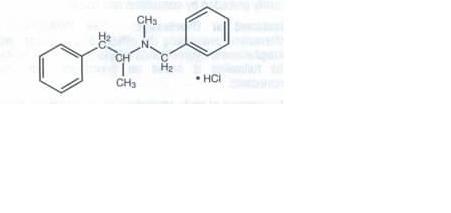
Benzphetamine hyrochloride tablets 50 mg contain the anorectic agent benzphetamine hydrochloride.Benzephet-amine hydrochloride is a white crystalline powder readily soluble in water and 95% ethanol. The chemical name
for benaphetamine hydrochloride isd-N,a-Dimethyl-N-(phenylmethyl)-benzeneethanamine hydrochloride and its
moleculas weight is 275.82.
The structural formula (dextro form) is represented as follows below:
Each Benzphetamine hydrochloride tablets 50 mg, for oral administration, contains 50 mg of benzphetamine hydrochloride.
Inactive ingredients: Calcium Stearate, Polyethylene Glycol, FD and C Yellow No. 6, Lactose Anhydrous, Sorbitol.
CLINICAL PHARMACOLOGY
Benzphetamine hydrochloride is a sympathomimetic amine with pharmacologic activity similar to the prototype drugs of this class used in obesity, the amphetamines. Actions include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class in which these phenomena have been looked for.
Drugs of this class used in obesity are commonly known as "anorectics" or "anorexigenics". It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions, or metabolic effects, may be involved.
Adult obese subjects instructed in dietary management and treated with "anorectic" drugs, lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is the greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an "anorectic" drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician-investigator, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas the studies cited are restricted to a few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered to be clinically limited.
INDICATION AND USAGE
Benzphetamine Hydrochloride Tablets are indicated in the management of exogenous obesity as a short term adjunct (a few weeks) in a regimen of weight reduction based on caloric restriction. The limited usefulness of agents of this class (see CLINICAL PHARMACOLOGY) should be weighed against possible risks inherent in their use such as those described below.CONTRAINDICATIONS
Benzphetamine Hydrochloride Tablets are contraindicated in patients with advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism, known hypersensitivity or idiosyncrasy to sympathomimetic amines, and glaucoma. Benzphetamine should not be given to patients who are in an agitated state or who have a history of drug abuse.
Hypertensive crises have resulted when sympathomimetic amines have been used concomitantly or within 14 days following use of monoamine oxidase inhibitors. Benzphetamine Hydrochloride Tablets should not be used concomitantly with other CNS stimulants.
Benzphetamine Hydrochloride Tablets may cause fetal harm when administered to a pregnant woman. Amphetamines have been shown to be teratogenic and embryotoxic in mammals at high multiples of the human dose. Benzphetamine Hydrochloride Tablets are contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
WARNINGS
When tolerance to the anorectic effect develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinuedPRECAUTIONS
GeneralInsulin requirements in diabetes mellitus may be altered in association with use of anorexigenic drugs and the concomitant dietary restrictions.
Psychological disturbances have been reported in patients who receive an anorectic agent together with a restrictive dietary regime.
Caution is to be exercised in prescribing amphetamines for patients with mild hypertension. The least amount feasible
should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
Information For Patients
Amphetamines may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery ordriving a vehicle; the patient should therefore be cautioned accordingly.
Drug Interactions
Hypertensive crises have resulted when sympathamimetic amines have been used concomitantly or within 14 days following use ofmonoamine oxidase inhibitors. Benzphetamine hydrochloride tablets should not be used concomitantly with other CNS stimulants.
Amphetamines may enhance the effects of tricyclic antidepressants.
Urinary alkalinizing agents increase blood levels and decrease excretion of amphetamines. Urinary acidifying
agents decrease blood levels and increase excretion of amphetamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies to evaluate the potential for carcinogenesis, mutagenesis or impairment of fertility have not been performed.ADVERSE REACTIONS
The following have been associated with the use of benzphetamine hydrochloride:Cardiovascular:
Palpitation, tachycardia, elevation of blood pressure. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
CNS:
Overstimulation, restlessness, dizziness, insomnia, tremor, sweating, headache; rarely, psychotic episodes at
recommended doses; depression following withdrawal of the drug.
Gastrointestinal:
Dryness of the mouth, unpleasant taste, nausea, diarrhea, other gastrointestinal disturbances.
Allergic:
Urticaria and other allergic reactions involving the skin.
Endocrine:
Changes in libido.
DRUG ABUSE AND DEPENDENCE
Benzphetamine is a controlled substance under the Controlled Substance Act by the Drug Enforcement Administration and has been assigned to Schedule IIIBenzphetamine hydrochloride is related chemically and pharmacologically to the amphetamines. Amphetamines and
related stimulant drugs have been extensively abused, and the possibility of abuse of benzphetamine Hydrochloride Tablets
should be kept in mind when evaluating the desirabilityof including a drug as part of a weight reduction program.
Abuse of amphetamines and related drugs may be associated with intense psychological dependence and severe social
dysfunction. There are reports of patients who have increased dosage to many times that recommended.
Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression;
changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include
severe dermatoses, marked insomnia, irritability, hyperactivity, and personality changes. The most severe manifestation
of chronic intoxication is psychosis, often clinically indistinguishable from schizophirenia.
OVERDOSAGE
Manifestations of Overdosage:Acute overdosage with amphetamines may result in restlessness, tremor, tachypnea, confusion, assaultiveness and panic states.
Fatigue and depression usually follow the central stimulation. Cardiovasculas effects include arrhythmias,
hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting,
diarrhea, and abdominal cramps. Hyperpyrexia and rhabdomyolysis have been reported and can lead to a number
of associated complications. Fatal poisoning is usually preceded by convulsion and coma.
Treatment of Overdosage:(see WARNINGS)
Information concerning the effects of overdosage with benzphetamine hydrochloride tablets is extremely limited. The following
is based on experience with other anorexiants.
Management of acute amphetamine intoxication is largely symptomatic and includes sedation with a barbiturate. If hypertension
is marked, the use of a nitrite or rapidly acting alpha receptor blocking agent should be considered.
Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard.
Acidification of the urine increases amphetamine excretion. The oral LD50 is 174 mg/kg in mice and 104 mg/kg in rats. The intraperitioneal LD50 in mice is 153 mg/kg.
DOSAGE & ADMINISTRATION
Benzphetamine Hydrochloride Tablets are supplied as follows:50 mg (peach, round, imprinted with BP 650, scored)
12634-118-56 Bottle of 56
12634-118-44 Bottle of 84
STORAGE AND HANDLING
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature].Dispense in a tight, light-resistant container as defined in the USP.
A Schedule CS-III controlled drug substance.
PACKAGE LABEL,PRINCIPAL DISPLAY PANEL
56 Tablets NDC 12634-118-56Benzphetamine Tablets USP
CIII
50mg
Rx Only
Each Tablet Contains:
Benzphetamine Hydrochloride 50mg
Usual Adult Dosage:
1/2 to 1 tablet one to three times daily. See insert for
full prescribing information.
Keep This and all Medication Out of Reach of Children.
Dispense in a tight, light-resistant container
as defined in the USP, using a child-resistant closure.
Manufactured by Tidor Pharma for
Boca Pharmacal, Inc.
Coral Springs, FL 33065
NDC 64376-650-01
Repackaged and Distributed by
Apotheca, Inc.
Phoenix, AZ 85006


PD-Rx Bottle Labels
| BENZPHETAMINE HYDROCHLORIDE
benaphetamine tablet |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA040747 | 12/09/2009 | |
| Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| PD-Rx Pharmaceuticals, Inc. | 156893695 | repack | |
Revised: 03/2010 PD-Rx Pharmaceuticals, Inc.