cis-mdp (medronate disodium) injection, powder, lyophilized, for solution
[CIS-US, Inc.]
DIAGNOSTIC FOR INTRAVENOUS USE
Rx Only
Description
CIS-MDP™ Kit for the Preparation of Technetium Tc 99m Medronate is a multidose reaction vial which contains the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Medronate Injection for diagnostic use by Intravenous injection.
Each 10mL multidose vial contains:
Medronic acid: 20 mg
Ascorbic acid: 1 mg
Stannous fluoride, SnF2: 0.13 mg (minimum)
Total tin (maximum, as stannous fluoride, SnF2): 0.38 mg
The pH is adjusted to 6.5 (6.3 to 6.7) with sodium hydroxide and/or hydrochloric acid prior to lyophilization. No bacteriostatic preservative is present in the vial. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture. The structural formula is:
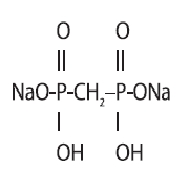
When a solution of sterile, non-pyrogenic, oxidant-free Sodium Pertechnetate Tc 99m Injection is added to the vial, the diagnostic agent, Technetium Tc 99m Medronate is formed for administration by intravenous injection. The pH of the reconstituted product is 5.4 to 6.8. The precise structure of Technetium Tc 99m Medronate Injection is not known at this time.
PHYSICAL CHARACTERISTICS:
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours1. The principal photon that is useful for detection and imaging studies is listed in Table 1.
| Radiation | Mean % per Disintegration | Mean Energy (keV) |
| Gamma-2 | 89.07 | 140.5 |
- 1
-
Kocher, DC: Radioactive Decay Data Tables, DOE/TIC-11026, 108, 1981.
EXTERNAL RADIATION:
The specific gamma ray constant for Tc 99m is 0.78 R/millicurie-hr at 1 cm. The first half-value layer is 0.017 cm of lead (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of the various thicknesses of Pb is shown in Table 2. To facilitate control of the radiation exposure from millicurie amounts of this radionuclide, the use of a 0.25 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1,000.
| Shield Thickness (Pb) cm | Coefficient of Attenuation |
| 0.017 | 0.5 |
| 0.08 | 10-1 |
| 0.16 | 10-2 |
| 0.25 | 10-3 |
| 0.33 | 10-4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
|
|||
| Hours | Fraction Remaining | Hours | Fraction Remaining |
| 0* | 1.000 | 7 | 0.447 |
| 1 | 0.891 | 8 | 0.398 |
| 2 | 0.794 | 9 | 0.355 |
| 3 | 0.708 | 10 | 0.316 |
| 4 | 0.631 | 11 | 0.282 |
| 5 | 0.562 | 12 | 0.251 |
| 6 | 0.501 | ||
Clinical Pharmacology
During the initial 24 hours following intravenous injection of Technetium Tc 99m Medronate, about 50% of each dose is retained in the skeleton, and about 50% is excreted in the urine. Upon intravenous injection, Technetium Tc 99m Medronate exhibits a specific affinity for areas of altered osteogenesis. In humans, blood levels fall to 4 to 10% of the injected dose by two hours post-injection and to 3 to 5% by three hours.
Uptake of Technetium Tc 99m Medronate Injection in bone appears to be related to osteogenic activity and to skeletal blood perfusion. The deposition in the skeleton is bilaterally symmetrical, with increased accumulation in the axial structure as compared to the appendicular skeleton. There is increased activity in the distal aspect on long bones as compared to the diaphyses.
Indications and Usage
Technetium Tc 99m Medronate Injection may be used as a bone imaging agent to delineate areas of altered osteogenesis.
Contraindications
None known.
Warnings
This class of compounds is known to complex cations such as calcium. Particular caution should be used with patients who have, or may be predisposed to hypocalcemia (i.e., alkalosis).
Preliminary reports indicate impairment of brain scans using Sodium Pertechnetate Tc 99m Injection which have been preceded by a bone scan using an agent containing stannous ions. The impairment may result in false-positive or false-negative brain scans. It is recommended, where feasible, that brain scans precede bone imaging procedures. Alternatively, a brain imaging agent such as Technetium Tc 99m Pentetate Injection may be employed.
Precautions
General
Contents of the vial are intended only for use in the preparation of Technetium Tc 99m Medronate Injection and are NOT to be administered directly to the patient.
Technetium Tc 99m Medronate Injection as well as other radioactive drugs must be handled with care, and appropriate safety measures should be used to minimize radiation exposure to the patient and clinical personnel consistent with proper patient management.
To minimize radiation dose to the bladder, the patients should be encouraged to drink fluids and to void immediately before the examination and as often thereafter as possible for the next 4 to 6 hours.
Technetium Tc 99m Medronate Injection should be formulated within six (6) hours prior to clinical use. Optimal imaging results are obtained 1 to 4 hours after administration.
The finding of an abnormal concentration of radioactivity implies the existence of underlying pathology, but further study is required to distinguish benign from malignant lesions.
The image quality may be adversely affected by obesity, old age, or impaired renal function.
The components of the kit are sterile and non-pyrogenic. It is essential to follow directions carefully and to adhere to strict aseptic procedures during preparation. Technetium Tc 99m labeling reactions involved depend on maintaining the stannous ion in the reduced state. Hence, Sodium Pertechnetate Tc 99m Injection containing oxidants should not be used.
The preparation contains no bacteriostatic preservative. Technetium Tc 99m Medronate Injection should be stored at 20-25ºC (68-77ºF) and discarded 6 hours after reconstitution. The solution should not be used if the contents are cloudy.
Vials are sealed under nitrogen: air or oxygen is harmful to the contents of the vials and the vials should not be vented.
The components of the CIS-MDP are supplied sterile and non-pyrogenic. Aseptic procedures normally employed in making additions and withdrawals for sterile, non-pyrogenic containers should be used during addition of the pertechnetate solution and the withdrawal of doses for patient administration.
Shielding should be utilized when preparing Technetium Tc 99m Medronate Injection.
No special handling is required for the non-radioactive drug product.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, mutagenesis, impairment of fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or whether Technetium Tc 99m Medronate Injection affects fertility in males or females. Mutagenesis studies have not been conducted.
Pregnancy
Category C
Animal reproduction and teratogenicity studies have not been conducted on Technetium Tc 99m Medronate Injection. It is also not known whether Technetium Tc 99m Medronate Injection can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Technetium Tc 99m Medronate Injection should be given to a pregnant woman only if clearly needed.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, on a woman of childbearing capability, should be performed during the first few (approximately 10) days following the onset of menses.
Nursing mothers
Technetium Tc 99m Medronate Injection is excreted in human milk during lactation; therefore, formula feeding should be substituted for breast feeding.
Pediatric Use
Safety and effectiveness in pediatric subjects have not been established.
Adverse Reactions
Several adverse reactions due to Technetium Tc 99m Medronate Injection have been reported. These were usually hypersensitivity reactions characterized by itching, various skin rashes, hypotension, chills, nausea and vomiting. There have also been rare cases of dizziness and asthenia associated with the use of Technetium Tc 99m Medronate.
Dosage and Administration
Shielding should be utilized when preparing Technetium Tc 99m Medronate Injection.
After preparation with oxidant-free Sodium Pertechnetate Tc 99m Injection, the suggested dose range of Technetium Tc 99m Medronate Injection in the average ADULT patient (70 kg.) is:
370-740 megabecquerels: (10-20 millicuries) given intravenously.
Imaging is optimal at 1 to 4 hours post Injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Radiation Dosimetry
The effective half-life was assumed to be the physical half-life for all calculated values. The estimated radiation absorbed doses to an average ADULT patient (70 kg) from an intravenous injection of a maximum of 740 megabecquerels (20 millicuries) of Technetium Tc 99m Medronate Injection are shown in Table 4.
|
|||
| Organ | (MGy/740 MBq) | (Rads / 20 mCi) | |
| Total Body | 1.3 | 0.13 | |
| Bone Total | 7.0 | 0.70 | |
| Red Marrow | 5.6 | 0.56 | |
| Kidneys | 8.0 | 0.80 | |
| Liver | 0.6 | 0.06 | |
| Bladder Wall | 2 hour void | 26 | 2.60 |
| 4.8 hour void | 62 | 6.20 | |
| Ovaries | 2 hour void | 2.4 | 0.24 |
| 4.8 hour void | 3.4 | 0.34 | |
| Testes | 2 hour void | 1.6 | 0.16 |
| 4.8 hour void | 2.2 | 0.22 | |
HOW SUPPLIED
The CIS-MDP Kit for the Preparation of Technetium Tc 99m Medronate Injection is supplied in kits of five (5) or thirty (30) sterile, non-pyrogenic vials. Each 10 mL multidose vial contains 20 mg medronic acid, 1 mg ascorbic acid, 0.13 mg minimum stannous fluoride (SnF2) and 0.38 mg maximum total tin, as stannous fluoride, SnF2 in lyophilized form. The pH is adjusted with sodium hydroxide and/or hydrochloric acid prior to lyophilization. The vial does not contain a preservative. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture. The pH of the reconstituted product is 5.4 to 6.8.
Kit Contents
Included in each five (5) vial kit is one (1) package insert and ten (10) radiation labels. Included in each thirty (30) vial kit is one (1) package insert and sixty (60) radiation labels.
Storage
Store the product as supplied at 20-25°C (68-77°F) [See USP]. After reconstitution store at 20-25°C (68-77°F) [See USP] (see DOSAGE AND ADMINISTRATION).
DIRECTIONS FOR PREPARATION OF TECHNETIUM Tc 99m MEDRONATE INJECTION:
Procedural Precautions
The lyophilized powder in the reaction vial is sterile and non-pyrogenic and does not contain a preservative. Shielded syringes and aseptic procedures normally employed in making additions and withdrawals from sterile, non-pyrogenic containers should be used during addition of pertechnetate solution to the reaction vial and the withdrawal of doses for patient administration.
If sodium pertechnetate Tc 99m must be diluted prior to injection into the reaction vial, only Sodium Chloride Injection USP 0.9% (without preservatives) should be used.
Technetium Tc 99m Medronate Injection is prepared from CIS-MDP by the following aseptic procedure:
- Waterproof gloves should be worn during the preparation procedure. Remove the plastic disk from the vial and swab the top of the vial closure with alcohol to sanitize the surface.
- Complete the radiation label and affix to the vial. Place the reaction vial in a suitable radiation shield.
- With a sterile, shielded syringe aseptically obtain 0.5-5 mL, of a suitable, oxidant-free, sterile, non-pyrogenic Sodium Pertechnetate Tc 99m Injection containing no more than 18.5 gigabecquerels (500 millicuries). Aseptically add the Sodium Pertechnetate Tc 99m Injection to the vial. Be sure to maintain a nitrogen atmosphere in the vial by not introducing air during reconstitution.
- Swirl the contents of the vial for one minute, and let stand for at least 10 minutes.
- Record time and date of preparation.
- The radiochemical purity of the prepared radiopharmaceutical should be checked prior to patient administration.
- Examine vial contents for particulates and discoloration prior to injection. Do not use if solution is cloudy.
- Withdrawals for administration must be made aseptically using a sterile shielded syringe and needle. Since the vials contain nitrogen to prevent oxidation of the complex, the vials should not be vented. If repeated withdrawals are made from a vial, the replacement of contents with air should be minimized.
- Use within six (6) hours of preparation. For optimum results, this time should be minimized. The vial contains no bacteriostatic preservative. After reconstitution store at 20-25°C (68-77°F)[See USP].
- The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
NDC# 045567-0040-1 (5 vial pack)
NDC# 045567-0040-2 (30 vial pack)
This reagent kit for the preparation of a radiopharmaceutical is approved for use by persons licensed pursuant to Section 120.533, Code of Massachusetts Regulation 105, or under equivalent license of the U.S. Nuclear Regulatory Commission or an Agreement State.
RM 2M-027
04/05
CIS-US, Inc.
10 DeAngelo Drive
Bedford, MA 01730
718-275-7129
| CIS-MDP (medronate disodium) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Revised: 10/2006CIS-US, Inc.